Figures & data

Figure 1 Macrophage polarization. Mφs can be roughly divided into two subtypes (M1 & M2) depending on different microenvironmental stimuli. M2 Mφs can be roughly divided into four subtypes (M2a, M2b, M2c, and M2d) depending on different microenvironmental stimuli. M1 Mφs are typically induced by IFN-γ/LPS while M2 Mφs are induced by IL-4/IL-10. M1 Mφ-sEVs secrete high levels of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-12, and IL-23, promoting inflammatory and cytotoxic responses. M2 Mφ-sEVs not only directly inhibit the expression of proinflammatory enzymes and cytokines, such as IL-12 and TNF-α, to achieve anti-inflammatory effects but also display higher levels of certain anti-inflammatory factors, such as IL-10 and TGF-β, thereby resolving deleterious inflammatory conditions.
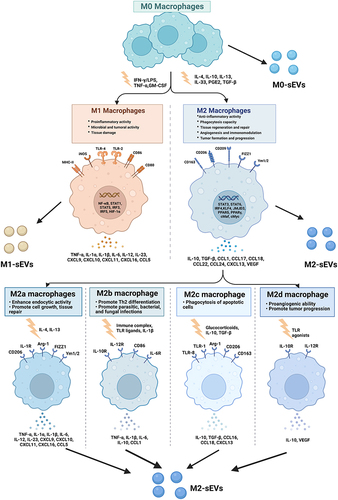
Figure 2 The formation of small extracellular vesicles derived from macrophages. The cytoplasmic membrane of Mφs initially invaginates to form endocytic vesicles, and multiple endocytic vesicles fuse to form early-sorting endosomes (ESEs). The ESEs then invaginate, encapsulating intracellular material in the process and further transforming into late-sorting endosomes (LSEs), which are known as multivesicular bodies (MVBs). MVBs then fuse with the cytoplasmic membrane and release EVs into the extracellular space.
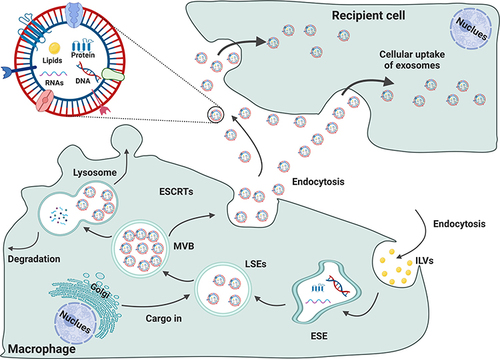
Table 1 Published Studies on Biogenesis of M0-Macrophage (M0-Mφ) Derived sEVs in Bone-Related Diseases
Table 2 Published Studies on Biogenesis of sEVs Derived from M1-Macrophage (M1-Mφ) in Bone-Related Diseases
Table 3 Published Studies on Biogenesis of sEVs Derived from M2-Macrophage (M2-Mφ) in Bone-Related Diseases
Figure 3 Published data on the biogenesis of sEVs derived from M0 macrophages in bone-related diseases.
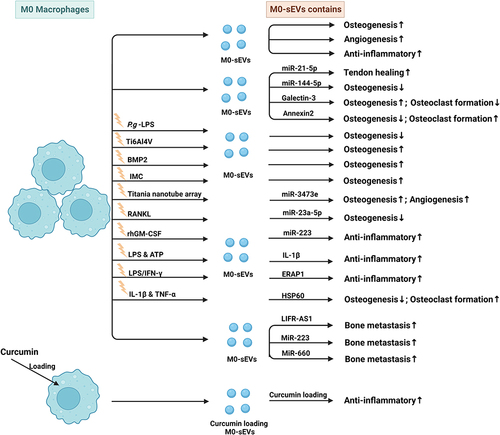
Figure 4 Published data on the biogenesis of sEVs derived from M1 macrophages in bone-related diseases.
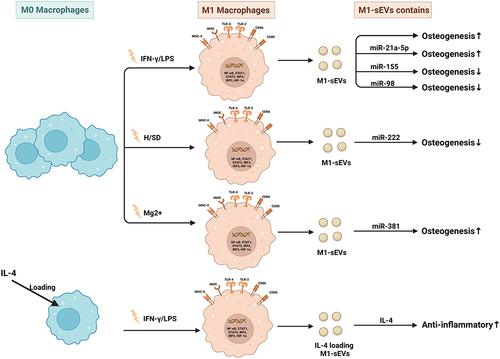
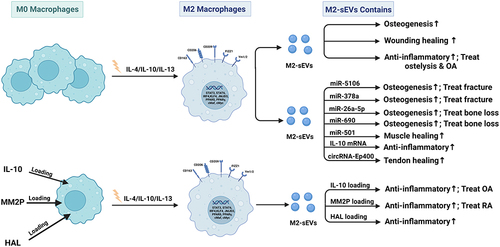
Figure 5 Published data on the biogenesis of sEVs derived from M2 macrophages in bone-related diseases.