Figures & data
Table 1 The Composition of Each Nanoemulsions
Table 2 The Fatty Acid Composition of G. bimaculatus Oil
Table 3 Biological Activities Related to Cosmeceutical Applications of G. bimaculatus Oil
Figure 2 Dose response curves on 2,2’-azino-bis(3-ethylbenzthia zoline-6-sulphonic acid (ABTS) inhibition (A), 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) inhibition (B), ferric reducing antioxidant power (C), and lipid peroxidation inhibition (D) of G. bimaculatus oil, ascorbic acid, and Trolox.
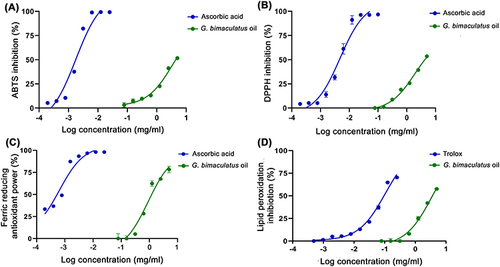
Figure 3 Dose response curves on tyrosinase inhibition when the substrate was L-tyrosine (A) and L-DOPA (B) of G. bimaculatus oil and kojic acid.
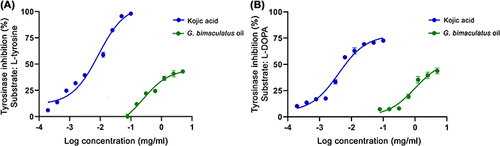
Figure 4 Dose response curves on inhibition of collagenase (A), elastase (B), and hyaluronidase (C) of G. bimaculatus oil, epigallocatechin gallate (EGCG), and oleanolic acid.
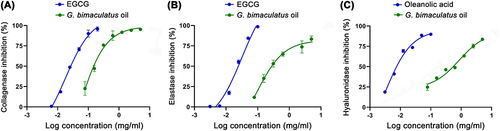
Figure 5 Cytotoxic effect of G. bimaculatus oil and its major fatty acid component, including linoleic acid, oleic acid, and palmitic acid, on immortalized human epidermal keratinocyte (HaCaT) cells (A) and human foreskin fibroblasts (Hs68) cells (B) after 48 h treatment. The data are demonstrated as mean ± SD (n = 3).
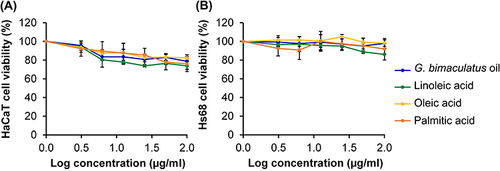
Figure 6 Effect of positive control (1% w/v sodium lauryl sulfate (SLS) aqueous solution), negative control (normal saline solution), G. bimaculatus oil and its major fatty acid component, including linoleic acid, oleic acid, and palmitic acid on the chorioallantoic membrane at 0, 5, and 60 min. The letter H represents hemorrhage, L represents vascular lysis, and C represents coagulation.
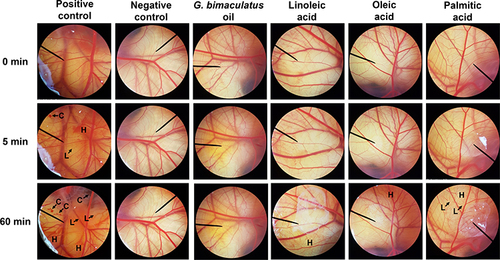
Figure 7 The external appearance of the emulsion of G. bimaculatus oil using different emulsifier systems has the hydrophilic–lipophilic balance (HLB) ranging from 8 to 12 after the preparation and after 24 h.

Figure 8 The external appearance of the emulsion of G. bimaculatus oil using the emulsifier systems with hydrophilic–lipophilic balance (HLB) of 9 (A), internal droplet size (B), polydispersity index (PDI) (C), and zeta potential (D) before and after an accelerated stability test in 8 heating-cooling cycles. The letters (a and b) indicate a statistically significant difference between nanoemulsion containing G. bimaculatus oil formulations F1, F2, and F3 (P<0.05).
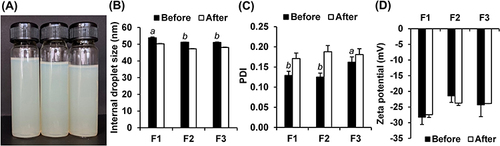
Figure 9 Transmission electron micrographs of nanoemulsion containing G. bimaculatus oil formulations F1 operating at 50k (A) and 40k (B) magnification.

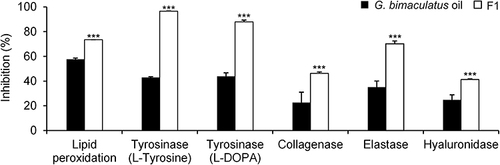
Figure 10 Inhibitory activities against lipid peroxidation, tyrosinase (when the substrate was L-tyrosine and L-DOPA), collagenase, elastase, and hyaluronidase of 1% w/w G. bimaculatus oil and nanoemulsion containing G. bimaculatus oil (F1). Asterisks (***) indicate a statistically significant difference between G. bimaculatus oil and F1 (P<0.01).