Figures & data
Figure 2 Release of DAMPs and binding of related receptors during ICD. DAMPs: CRT, ATP, ANXA1, HMGB1; Receptors: LRP1, P2RY2/P2RX7, FPR1, TLR2/ TLR4, LOX1.
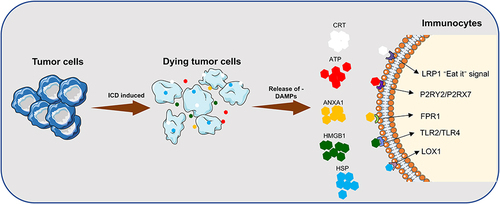
Figure 3 Release of ICD-associated DAMPs from tumor cells and their catalytic “functionalization” to immune cells.
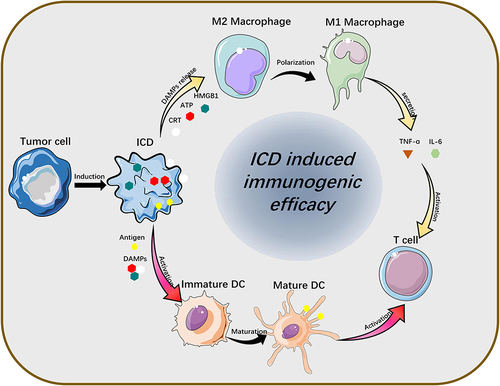
Figure 4 Schematic illustration of the DOX@LINV for tumor inhibition based on immunogenic tumor nanovesicles (TNVs) synergistic with the DOX-induced ICD effect. (A) Preparation of DOX@LINV by the confusion of TNVs with artificial liposomes. (B) Mechanism of immunochemotherapy based on the DOX@LINV for tumor suppression. (C) SDS–PAGE of B16F10 cells, B16F10-derived nanovesicles, and LINVs. (D) Western blotting of specific antigen preservation on TNVs and LINVs. (E) Representation flow cytometry plots of mature DCs after a 24 h treatment. (F) ATP, (G) HMGB-1, and (H) CRT release from B16F10 cells analyzed with the ELISA kit after 24 h of incubation (n = 3, *p < 0.05; **p < 0.01). (I) CRT expression after treatment evaluated by CLSM. Scale bar: 50 μm. Reprinted from Hu M, Zhang J, Kong L, et al . Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer ImmunochemotherapyImmunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. Acs Nano. 2021;15(2):3123–3138. Copyright © 2021, American Chemical Society.Citation80
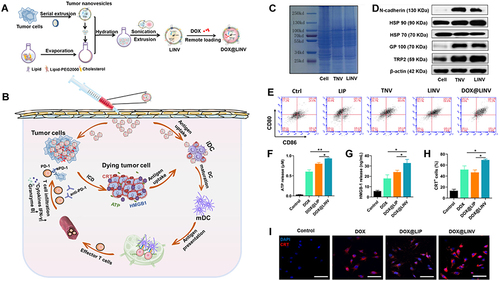
Figure 5 Schematic illustration of the nano-ligand polymer for radio sensitization by amplifying intracellular oxidative stress. (A) Schematic diagram of the preparation of nano-ligand polymer. (B) H@Gd-NCP H@Gd-NCPs enhance the mechanism of checkpoint blockade immunotherapy. (C) Immunofluorescence of CRT antibody. (D) Western blot of HMGB1. (E) Detection of HMGB1 release by ELISA kit (n = 3, ***p = 0.0001, ***p = 0.0001, **p = 0.0014). (F) Detection of ATP secretion by luciferin-based ATP assay kit (n = 3, ***p = 0.0001, ***p = 0.0003). (G) Flow cytometry analysis of DCs maturation in tumor-draining lymph nodes (TDLNs) after radiotherapy (0 or 6 Gy × 1, n = 6, **p = 0.0063). N.S. represented non-significance, and **p < 0.01, ***p < 0.001. Reprinted from Huang Z, Wang Y, Yao D, Wu J, Hu Y, Yuan A. Nanoscale coordination polymers induce immunogenic cell death by amplifying radiation therapy mediated oxidative stress. Nat Commun. 2021;12(1):145. Creative Commons.Citation93
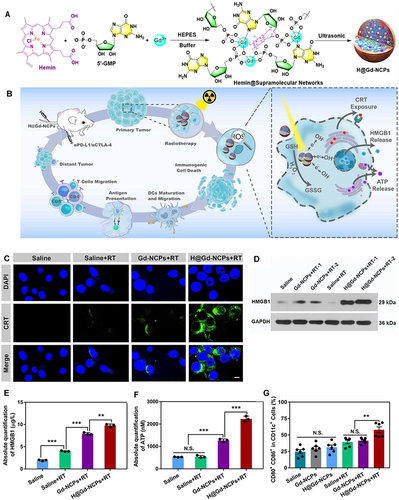
Figure 6 Preparation and mechanism of pRNVs/HPPH/IND. (A) Construction of pH-Responsive Nanovesicles (pRNVs/HPPH/IND) via Co-assembly of HPPH, IND, and pH-Responsive Polypeptide. (B) Single low dose i.v. Injection of pRNVs/HPPH/IND to promote host immunity and induce tumor cell death. CRT release from B16F10 cells after 24 h incubation analyzed with CLSM (C) and flow cytometry (D) Scale bar: 40 µm. (E) Apoptosis in B16F10 cells induced by different formulation via flow cytometry Symbol (+) denotes laser irradiation at 671 nm (100 mW/cm2, 1 min). Reprinted from Yang W, Zhang F, Deng H, et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano. 2020;14(1):620–631. Copyright © 2020, American Chemical Society.Citation104
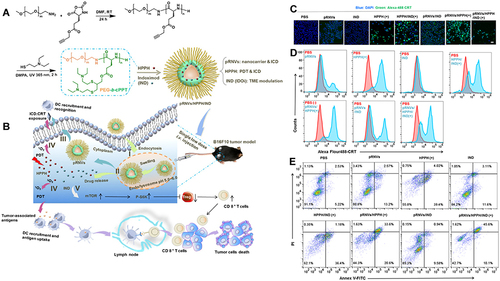
Figure 7 Preparation and mechanism of APNA. (A) Chemical structure of pBODO-PEG-VR and preparation of APNA. (B) Mechanism of anti-tumor immune response by APNA-mediated NIR-II photothermal immunotherapy. (C) Immunofluorescent images of Cas-3 (green), HMGB1 (green), and CD80/CD86 (Orange) in tumor sections at different photothermal depths after different treatments. Nuclei staining indicated by DAPI (blue). Scale bar: 20 µm. (D) DC maturation (gated on CD11c+ DCs) in tumor-draining lymph nodes from mice after different treatments. (E) Representative flow cytometry plots of CD8+ T cells and CD4+ T cells in tumor-infiltrating CD45+ lymphocytes in primary tumors from mice after various treatments. (F) Representative flow cytometry plots of CD8+ T cells and CD4+ T cells in tumor-infiltrating CD45+ lymphocytes in distant tumors from mice after various treatments. Reprinted from Jiang Y, Huang J, Xu C, Pu K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat Commun. 2021;12(1):742. Creative Commons.Citation111
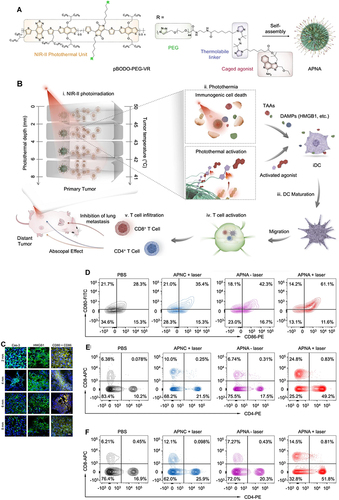
Figure 8 (A) Mechanism of the FVIO-mediated mild magnetic hyperthermia can activate the host immune systems and efficiently cooperate with PD-L1 blockade to inhibit the potential metastatic spreading as well as the growth of distant tumors. (B and C) Quantification of CRT exposure on the surface after treatment by flow cytometry (***p < 0.001). (D) Quantification of CRT exposure on the surface by RT-PCR analysis (*p < 0.05, **p < 0.01, ***p < 0.001). (E) Representative multispectral fluorescence images of distant tumors after treatment. Scale bar: 100 μm. Reprinted from Liu X, Zheng J, Sun W, et al. Ferrimagnetic Vortex Nanoring-Mediated Mild Magnetic Hyperthermia Imparts Potent Immunological Effect for Treating Cancer Metastasis. ACS Nano. 2019;13(8):8811–8825. Copyright © 2019, American Chemical Society.Citation116
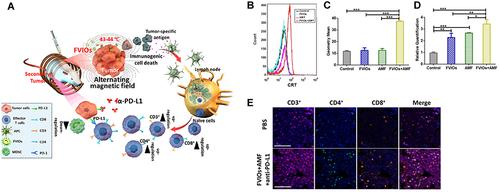
Table 1 Preclinical Studies of Nanocarrier-Mediated ICD for Melanoma Treatment
Figure 9 Overview of the literature visualization analysis on ICD induced therapies on melanoma in the last five years from the Web of Science. (A) Network visualization. (B) Density visualization.
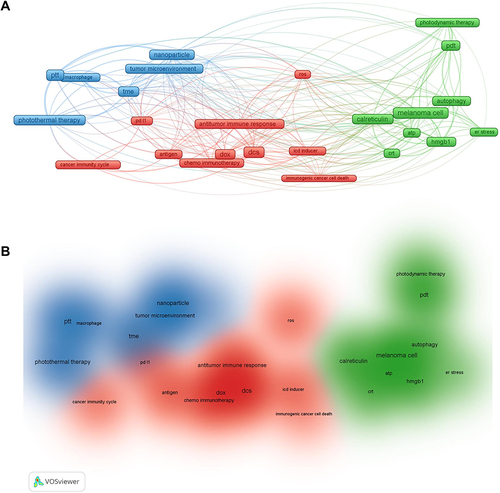
Table 2 Clinical Studies of Nanocarrier-Mediated ICD for Melanoma Treatment