Figures & data
Figure 1 Challenges of conventional AD drugs. (1) Drugs are often degraded during intrahuman circulation due to their poor stability or stimulation by factors within the body; (2) the special composition of the BBB sets up natural barriers for AD drug delivery, greatly reducing the penetration rate of drugs; and (3) drugs that cross the BBB into the brain are randomly distributed to the whole brain, lacking targeted therapeutic effects. Image created with Biorender.com.
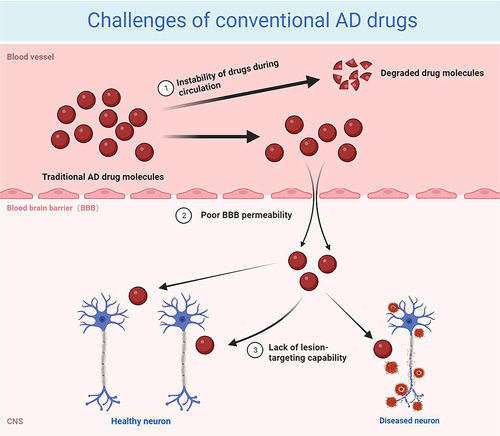
Figure 2 Schematic snapshot of the blood–brain barrier. Reprinted from Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4(2):78–82. Creative Commons.Citation14
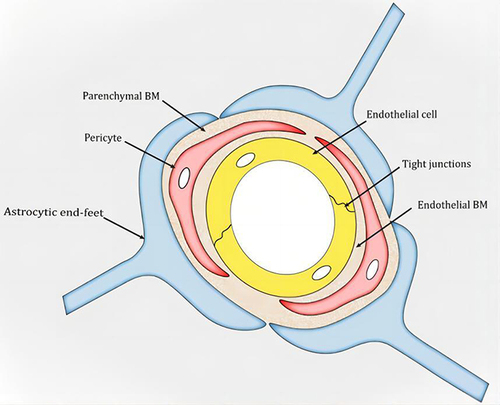
Table 1 Characters of Biomembrane-Derived Nanoparticles for AD Treatment
Table 2 Summary of the Advantages and Disadvantages of Different Methods of Engineering Biomembranes
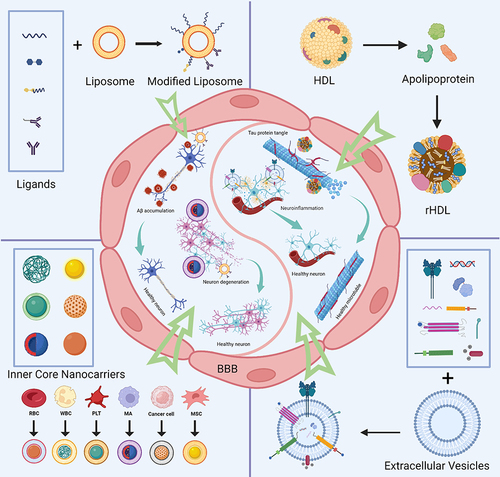
Figure 3 Schematic diagram and advantageous study of liposomes in AD-targeted therapy. (A) Schematic diagram of liposome-targeted therapy for AD through different pathways. Image created with Biorender.com. (B) Schematic illustration of the strategy for improving AD-related pathology by Tf-modified osthole liposomes. Reprinted with permission from Dove Medical Press. Kong L, Li XT, Ni YN, et al. Transferrin-modified osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomedicine. 2020;15:2841–2858.Citation28 (C) Real-time imaging observation after intravenous administration of varying liposomal formulations in APP/PS-1 mice (n=3). Reprinted with permission from Dove Medical Press. Kong L, Li XT, Ni YN, et al. Transferrin-modified osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomedicine. 2020;15:2841–2858.Citation28 (D–F) The biosafety of Tf-Pep63-Lip in vivo and in vitro: Tf-Pep63-Lip and Tf-Lip do not induce adverse effects in vivo and in vitro. Reprinted from Yang X, Li X, Liu L, et al. Transferrin-Pep63-liposomes accelerate the clearance of Abeta and rescue impaired synaptic plasticity in early Alzheimer’s disease models. Cell Death Discov. 2021;7(1):256. Creative Commons.Citation29
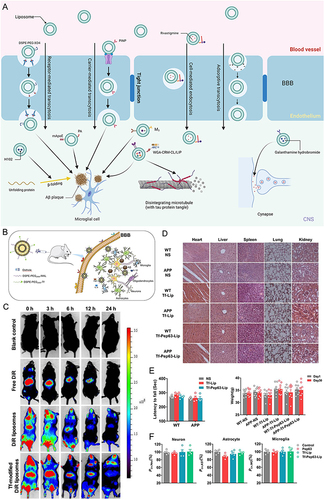
Figure 4 Schematic diagram and advantageous study of HDL-based nanoparticles delivering drugs in AD-targeted therapy. (A) Schematic diagram of HDL nanoparticles (exemplified by APLN/MB) delivering drugs through the BBB via receptor-mediated endocytosis with the help of LRP1 and other receptors to target AD. Reprinted from Han G, Bai K, Yang X, et al “Drug-Carrier” synergy therapy for amyloid-beta clearance and inhibition of tau phosphorylation via biomimetic lipid nanocomposite assembly. Adv Sci. 2022;9(14):e2106072. © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. Creative Commons.Citation76 (B) After α-M loading, apolipoprotein E (ApoE)-reconstituted high-density lipoprotein nanocarriers (ANCs) can inhibit the formation of Aβ oligomers and fibrils and hasten Aβ cellular degradation. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.Citation75 (C) Brain distribution of ANC following intravenous administration. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.Citation75 (D) ANC-α-M decreased amyloid deposition and attenuated microgliosis in the brains of SAMP8 mice. (**p < 0.01, ***p < 0.001, significantly different from that in SAMP8 mice treated with NS; #p < 0.05, ###p < 0.001, significantly different from that in the SAMR1 mice treated with NS. Black arrows point to amyloid plaques and white arrows point to CD45-positive activated microglia.) Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.Citation75 (E) In vivo accumulation of ANC (red) surrounding Aβ aggregates (green) in the cortex and hippocampus following intravenous administration. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.
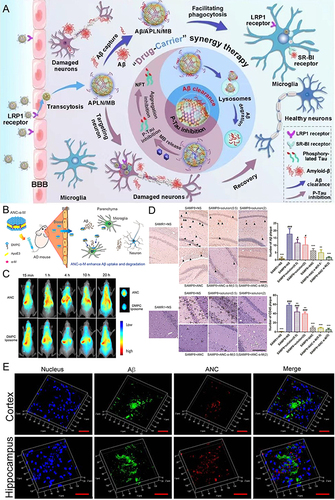
Table 3 Summary of the Features Between Different Cell Membrane-Based Nanoparticles
Figure 5 Schematic diagram and advantageous study of cell membrane-based nanoparticles delivering drugs in AD-targeted therapy. (A) Schematic diagram of cell membrane-based nanoparticles as drug carriers for targeted treatment of AD. Image created with Biorender.com. (B) Compared with the saline (control) group, no indicators of damage were observed for these organs after treatment. (C) The most intense distribution in the brain was displayed in the DIR-tagged RVG/TPP-MASLNs-treated mice and was further confirmed by the fluorescence intensity identified in the isolated brain homogenate. (*p < 0.05 compared with MASLNs.) (D) Administration of GS-loaded formulations slowed the alleviation of degenerative alterations of hippocampal neurons in AD mice to different extent. (Red arrows point to the damaged neurons.) Adapted with permission from KeAi Publishing. Han Y, Gao C, Wang H, et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–542.Citation100
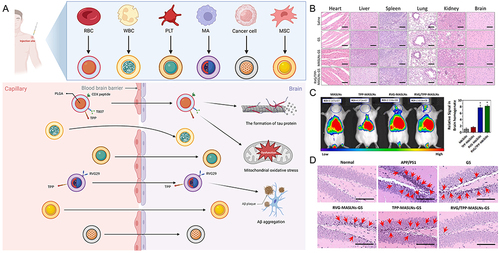
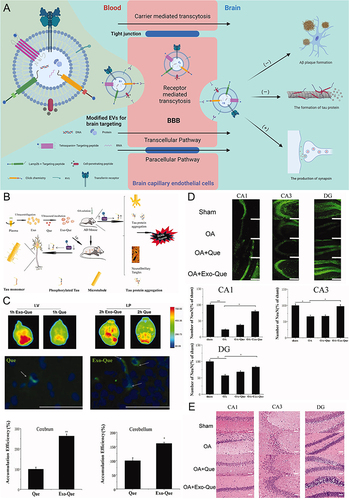
Figure 6 Schematic diagram and advantageous study of extracellular vesicles delivering drugs in AD-targeted therapy. *(A) Schematic diagram of variously modified extracellular vesicles capable of crossing the BBB to target AD. Image created with Biorender.com. (B) Exo-Que enhanced the bioavailability, achieved brain targeting and improved cognitive and functional symptoms in OA-induced AD mice. (C) Exo-Que significantly enhanced accumulation of Que in brain and highly enhanced fluorescence of Que was observable in brain in Exo-Que-treated group. (*p < 0.05, **p < 0.01.) (D) Compared with OA and OA + Que group, Exo-Que significantly increased the number of NeuN-positive neuron cells in hippocampal CA1 region, CA3 region and DG region. (*p < 0.05, **p < 0.01.) (E) H&E staining results showed that the neuronal layers in the hippocampus showed integrity and orderliness maintaining intact neuronal cells structure with clear line and abundant cytoplasm in OA + Exo-Que group. Reprinted from Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745–755. Creative Commons.