Figures & data
Figure 1 Pericytes declined in age-related bone resorption and bone resorption caused by estrogen deficiency (A) Representative immunostaining images of femurs from OVX and female WT mice. NG2+ (red) pericytes, endomucin+ (green) endothelial cell, and nuclear (blue) DAPI staining. Scale bar, 50 μm. (B) Quantification of NG2+ pericyte number in subepiphyseal zone of femurs from OVX and female WT mice (n = 6). (C) Representative TRAP staining images of femurs from OVX and female WT mice. Scale bar, 100 μm (lower panels); 500 μm (upper panels). (D) Quantification of osteoclast surface per bone surface (Oc.S/BS) and osteoclast number per bone perimeter (Oc.N/B.Pm) in subepiphyseal zone of femurs from OVX and female WT mice (n = 6). (E) Analysis of the Pearson correlation between NG2+ cell number and Oc.N/B.Pm in femurs from female wild-type mice of different ages (6, 12 and 18 months). Data are presented as the mean ± S.E.M. P-values < 0.05 were considered statistically significant, as follows: ***P < 0.001; ****P < 0.0001.
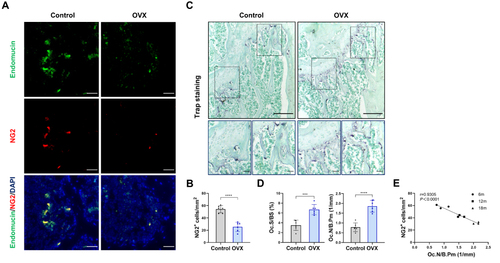
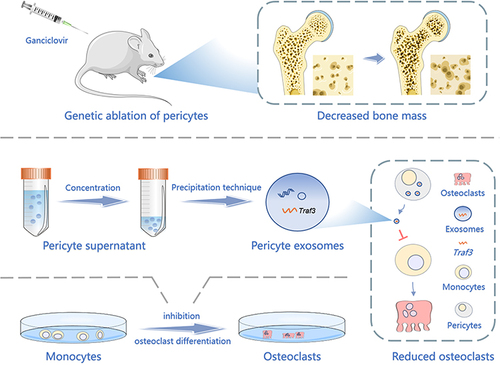
Figure 2 Genetic ablation of pericytes led to reduced bone mass (A) Schematic of NG2-tk mice modeling. Six-week-old female NG2-tk mice were intraperitoneally injected with GCV (50 mg/kg) once a day for 30 consecutive days in order to delete NG2+ pericytes. (B) Representative micro-CT images displaying the three-dimensional architectures of femurs from WT mice and NG2-tk mice (after GCV treatment). Scale bar, 1mm (left panels); 500 μm (right panels). (C) Micro-CT analysis of BV/TV, SMI, Tb.Th, and Tb.N in femurs of WT mice and NG2-tk mice (after GCV treatment, n = 6). (D) Representative H&E staining images of femurs from WT mice and NG2-tk mice (after GCV treatment). Scale bar, 500 μm. (E) Quantification of bone volume fraction (BV/TV) in femurs from WT mice and NG2-tk mice (after GCV treatment, n = 6). Data are presented as mean ± S.E.M. P-values < 0.05 were considered statistically significant, as follows: *P < 0.05; ***P < 0.001; ****P < 0.0001.
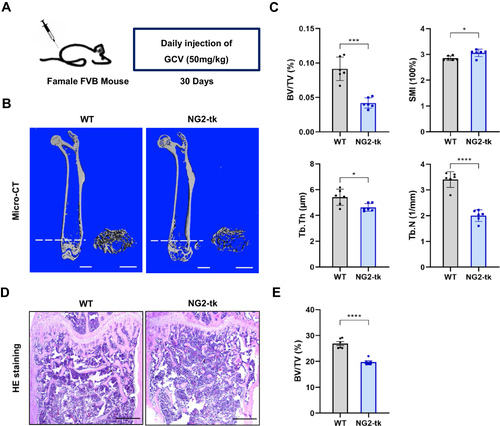
Figure 3 Genetic ablation of pericytes activated bone resorption (A) Representative immunostaining images of femurs from WT mice and NG2-tk mice (after GCV treatment). NG2+ (red) pericytes, endomucin+ (green) endothelial cell, and nuclear (blue) DAPI staining. Scale bar, 50 μm. (B) Quantification of NG2+ pericyte number in subepiphyseal zone of femurs from WT mice and NG2-tk mice (after GCV treatment, n = 6). (C) Representative TRAP staining images of mouse femurs from WT mice and NG2-tk mice (after GCV treatment). Scale bar, 500 μm (upper panels); 100 μm (lower panels). (D) Quantification of osteoclast surface per bone surface (Oc.S/BS) and osteoclast number per bone perimeter (Oc.N/B.Pm) in subepiphyseal zone of femurs from WT mice and NG2-tk mice (after GCV treatment, n = 6). Data are presented as mean ± S.E.M. P-levels < 0.05 were considered statistically significant, as follows: **P < 0.01; ****P < 0.0001.
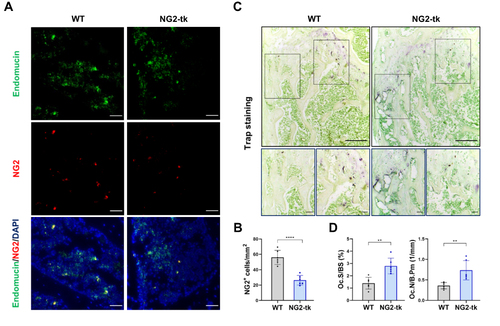
Figure 4 Traf3 in PC-EVs mediates the inhibition of osteoclast differentiation by pericytes (A) The images of PC-EVs under TEM. Bar, 100 μm. (B) NTA results of PC-EVs. (C) The characterization of PC-EVs by Western blotting. (D) Representative TRAP staining images of monocytes after 5 days of osteoclast differentiation. RAW264.7 cells were induced with a medium containing RANKL (100 ng/mL) and non-contact co-cultured with PC-EVs (5–10 μg/mL). Scale bar, 100 nm. (E) Quantification of osteoclast number (Oc.N) and area of osteoclast (area of oc) of monocytes after 5 days of osteoclast differentiation (n = 6). (F) GO analysis map of total RNA sequencing of PC-EVs. (G) The coding gene of osteoclast inhibitory protein. Data are presented as mean ± S.E.M. P-levels < 0.05 were considered statistically significant, as follows: ****P < 0.0001.
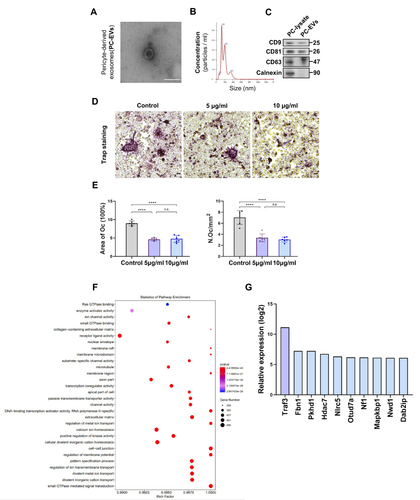
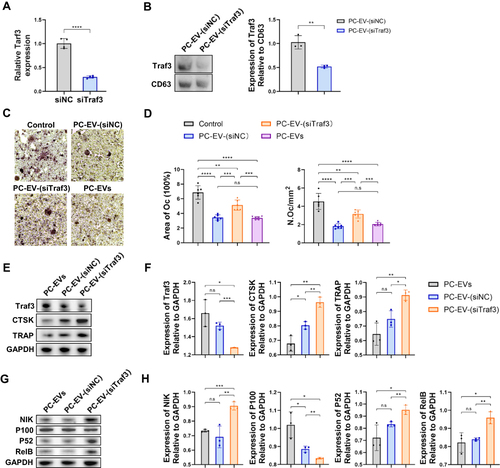
Figure 5 Traf3 inhibits osteoclast differentiation through the non-canonical NF-κB pathway (A) Relative mRNA expression of Traf3 in vascular pericytes transfected with Traf3 siRNA (n = 4). (B) Representative blots of exosome-derived TRAF3 and CD63, and quantification of the ratio of TRAF3/CD63 (n = 3). PC-EV-(siNC), exosomes generated from pericytes treated with NC siRNA. PC-EV-(siTraf3), exosomes generated from pericyte treated with Traf3 siRNA. (C) Representative TRAP staining images of monocytes after 5 days of osteoclast differentiation. RAW264.7 cells were induced with a medium containing RANKL (100 ng/mL) and non-contact co-cultured with exosomes (5 μg/mL). Bar, 100μm (D) Quantification of osteoclast number (Oc.N) and area of osteoclast (area of oc) of monocytes after 5 days of osteoclast differentiation (n = 6). (E) Representative blots of TRAF3, TRAP, and CTSK of monocytes after 5 days of osteoclast differentiation. (F) Quantification of the ratio of TRAF3, TRAP, and CTSK/GAPDH in monocytes after 5 days of osteoclast differentiation (n = 3). (G) Representative blots of NIK, P100, P52, and RElB in monocytes after 5 days of osteoclast differentiation. (H) Quantification of the ratio of NIK, P100, P52, and RElB in monocytes after 5 days of osteoclast differentiation (n = 3). Data are presented as mean ± S.E.M. P-levels < 0.05 were considered statistically significant, as follows: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.