Figures & data
Figure 1 Functions of DCs in autoimmunity and tolerance. DCs interact with T cells through MHC-I and -II. Activated pro-inflammatory DCs presenting autoantigens that trigger autoreactive killing CD8+ and CD4+ helper T cells. Tolerant DCs (Tol DCs) express different co-stimulatory receptors and cytokines that induce Tregs and deactivate autoreactive CD4+ and CD8+ T cells. Data from Cifuentes-Rius et al.Citation11
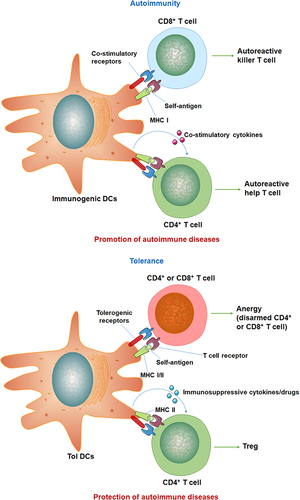
Figure 2 The nanomaterials used as the nanovaccines. Different nanomaterials can provide different functions of nanovaccines. Commonly used nanomaterials include inorganic nanoparticles (such as gold nanoparticles, carbon nanoparticles, silicon nanoparticles), polymer nanoparticles, liposome nanoparticles, and biomembrane-based nanoparticles. Nanovaccines can be captured by major histocompatibility MHC-II molecules in APCs and presented to CD4+ helper T (Th) cells. T cells that receive the antigen signal and further differentiate into Th1 or Th2 cells. The antigens presented by MCH-I molecules in the cytoplasm of APCs can activate cytotoxic CD8+ T lymphocytes (CTL) to directly kill infected cells.
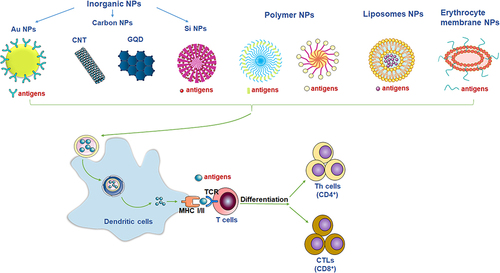
Table 1 Application of Nanovaccines in the Treatment of Autoimmune Diseases
Figure 3 Loaded with autoantigens to generate tolerant dendritic cells. AbaLDPN-MOG nanovaccine induced tolerogenic DCs. (A) The composition of AbaLDPN-MOG tolerogenic nanovaccine, and the mechanism of antigen-specific tolerance induction in lymph nodes. (B) The nanoparticles induced tolerogenic DC and detected the expression of related CD molecules and inflammatory factors. All statistical data are presented as mean ± SD (n = 5; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Adapted with permission from Park J, Le QV, Wu Y, Lee J, Oh YK. Tolerogenic nanovaccine for prevention and treatment of autoimmune encephalomyelitis. Adv Mater. 2023;35(1):e2202670;Citation25 © 2022 Wiley-VCH GmbH.
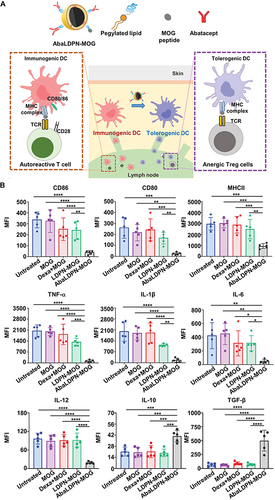
Figure 4 Pretreatment with αDCIR2-PLP139–151 mAb reduces activity of pathogenic T helper cells. The numbers of IL-17 producing cells (pathogenic Th17) and IFN-γ producing cells are significantly reduced in mice treated with αDCIR2-PLP139–151 mAb. All statistical data are presented as mean ± SD (n =2; *p < 0.05, **p < 0.01, ****p = 0.0001). Adapted with permission from Tabansky I, Keskin DB, Watts D et al. Targeting DEC-205(-)DCIR2(+) dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Molecular medicine. 2018;24(1):17.Citation79 Creative Commons.
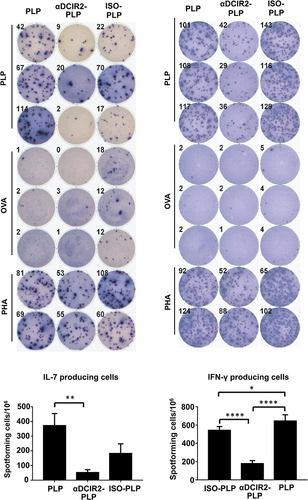
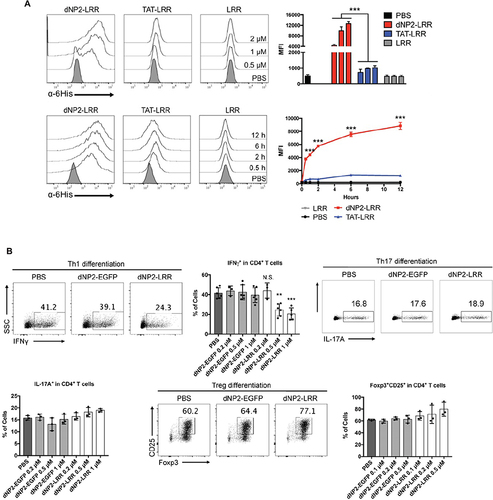
Figure 5 Cell-penetrating peptide-modified nanovaccines can improve antigen delivery efficiency. (A) The intracellular delivery efficiency of dNP2-LRR is increased compared with LRR alone. Reproduced with permission from Koo JH, Kim DH, Cha D, Kang MJ, Choi JM. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics. 2020;10(7):3138–3150.Citation89 Creative commons. (B) dNP2-LRR inhibits T cell activation and specifically inhibits Th1 differentiation. n=3 per group and error bars indicate S.D. Reproduced with permission from Koo JH, Kim DH, Cha D, Kang MJ, Choi JM. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics. 2020;10(7):3138–3150.89 Creative commons. **P<0.01 and ***P<0.001. N.S., not significant.