Figures & data
Figure 1 Schematic diagram of a standard second-generation CAR-T cell structure. A chimeric antigen receptor (CAR) has a heavy chain variable region (VH) and a light chain variable region (VL) in the sequence of an antibody against a target antigen. The hinge region links the transmembrane domain to the intracellular co-stimulatory domain and the CD3 signaling domain. If the scFv identifies the target antigen, it stimulates the signaling and co-stimulatory domains in order to promote sustained T cell proliferation and effector functions.
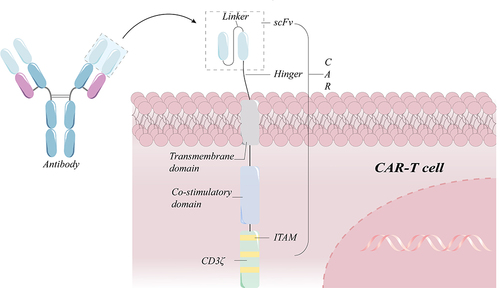
Figure 2 Biogenesis and killing mechanisms of exosomes released from CAR-T cell. The proteins on the surface of CAR-T cells enter the cell through the plasma membrane invagination and then can exchange cargo within the cell, followed by the transition of early sorters and late sorters, and finally form multivesicular bodies (MVBs), part of which enter the lysosome to be degraded and another part of which docks on the side of the plasma membrane under the action of docking proteins and is then expelled by the cell membrane, thereby releasing exosomes.Citation11 The exosomes generated by CAR-T cells not only have CAR molecules imbedded on their surface, but they also include cytotoxic particles that can precisely target tumor cells and then be absorbed to release granzyme and trigger endogenous apoptosis.
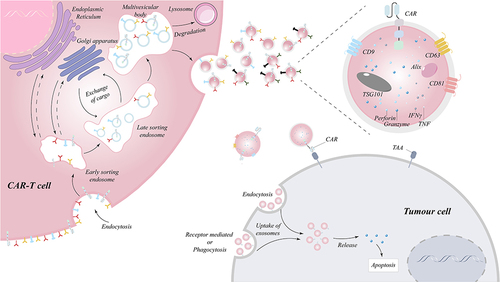
Figure 3 Mechanism of tumor relapse in target antigen-negative tumors caused by target antigen positivity and target antigen loss. (a) Fewer CAR-T cells may be responsible for tumor cell resistance to treatment. (b) Due to gene mutations or variable splicing, target antigens become unrecognized. (c) The absence of chaperone proteins results in improper protein maturation and membrane transport, which manifests as antigen loss. (d) Rearrangement of genes causes rearrangement of intracellular proteins, result in the loss of the target antigen.
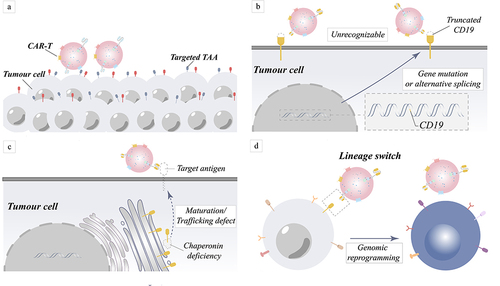
Figure 4 Mechanism of tumor relapse due to down-regulation of target antigen recognition. (a) Trogocytosis decreases the number of tumor antigens by transferring antigens from the surface of tumor cells to the surface of lymphocytes. (b) Masking of target antigens leads to down-regulation of recognition by CAR molecules.
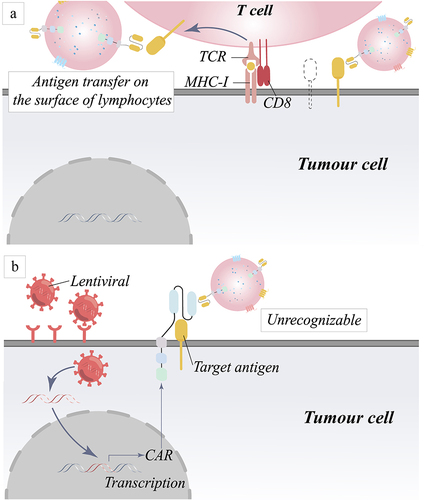
Figure 5 Schematic representation of the mechanism for addressing targeting challenges by artificially modifying proteins on the surface of tumor cells. (a) An approach for the precise identification and destruction of tumor cells based on the ability of liposomes to fuse cell membranes and the coating of tumor cells with artificial proteins. (b) A technique to altering tumor cell surface proteins for specific identification and death of tumor cells is reported, based on the premise that pHLIPs can be integrated into cells in an acidic environment generated by tumor cells.
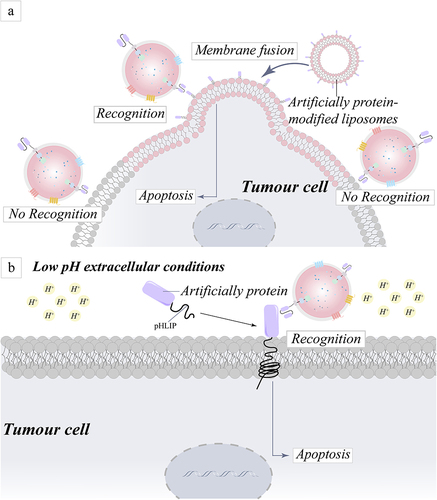
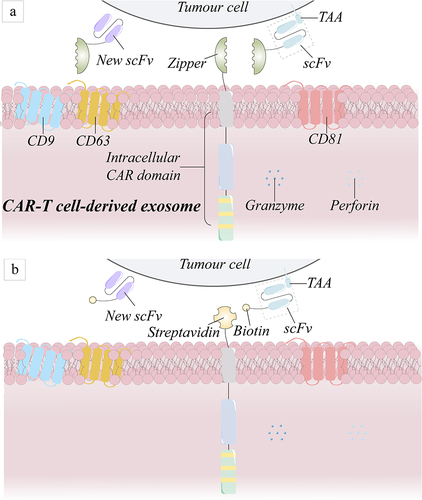
Figure 6 Schematic diagram of the general-purpose modular CAR structure. (a) The leucine zipper joins the scFv segment to produce a full CAR structure, whereas its receptor connects to the intracellular structural domain of the CAR structure. (b) The scFv fragment is modified by biotin, and streptavidin is attached to the intracellular structural domain of the CAR molecule, forming a complete CAR once the two are combined.