Figures & data
Figure 1 Chemical structure of docetaxel (DTX) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DIR).
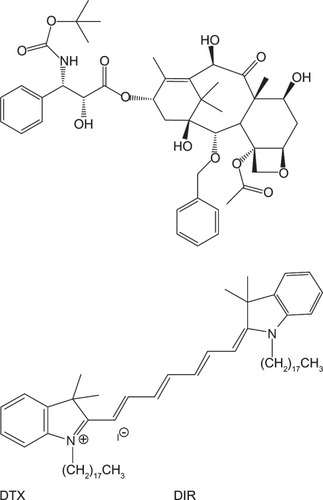
Figure 2 Synthetic route of poly(lactide)-D-α-tocopheryl polyethylene glycol 1000 succinate (PLA-TPGS).
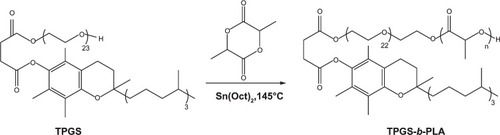
Figure 3 (A and B) Schematic diagram of Shirasu porous glass (SPG) membrane emulsification. (A) SPG membrane-emulsification apparatus; (B) membrane-emulsification principle.
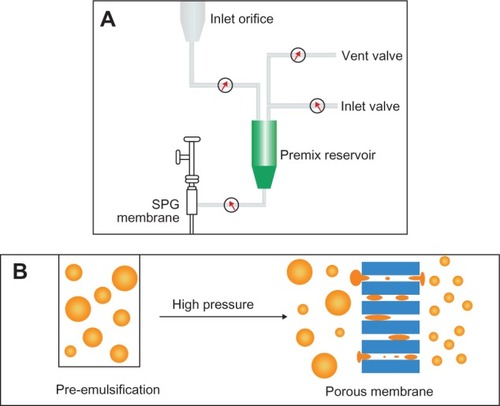
Figure 4 Hydrogen-1 nuclear magnetic resonance spectrum of poly(lactide)-D-α-tocopheryl polyethylene glycol 1,000 succinate.
Abbreviation: ppm, parts per million.
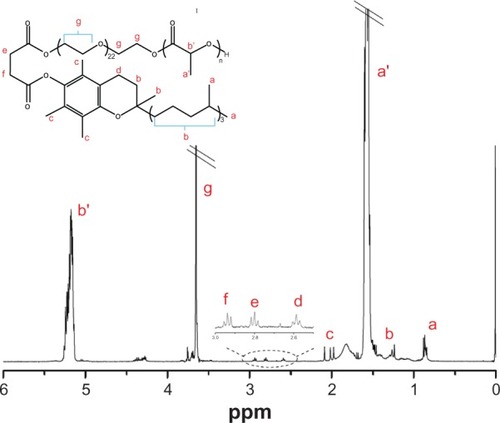
Table 1 Effects of surfactant type and surfactant concentration in aqueous phase in fabricating DTX-loaded PLA-TPGS nanoparticles on size, PDI, EE, and DLE
Table 2 Effects of volumetric ratio of organic to aqueous phase in fabricating DTX-loaded PLA-TPGS nanoparticles on size, PDI, EE, and DLE
Table 3 Effects of the transmembrane cycles in fabricating DTX-loaded PLA-TPGS nanoparticles on size, PDI, EE, and DLE
Table 4 Effects of the transmembrane pressure in fabricating DTX-loaded PLA-TPGS nanoparticles on size, PDI, EE, and DLE
Table 5 Effects of membrane-pore size in fabricating DTX-loaded PLA-TPGS nanoparticles on size, PDI, EE, and DLE
Table 6 Effects of PLGA and PLA-TPGS copolymer in fabricating DTX-loaded nanoparticles on size, PDI, EE, and DLE
Table 7 Properties of PLA-TPGS nanoparticles on a pilot scale
Figure 5 Scanning electron microscopy image of poly(lactide)-D-α-tocopheryl polyethylene glycol 1,000 succinate nanoparticles.

Figure 6 Cumulative release profiles of docetaxel-loaded poly(lactide)-D-α-tocopheryl polyethylene glycol 1,000 succinate (PLA-TPGS) nanoparticles (NPs) in phosphate-buffered saline (pH = 7.4) at 37°C.
Abbreviation: PLGA, poly(lactic-co-glycolic acid).
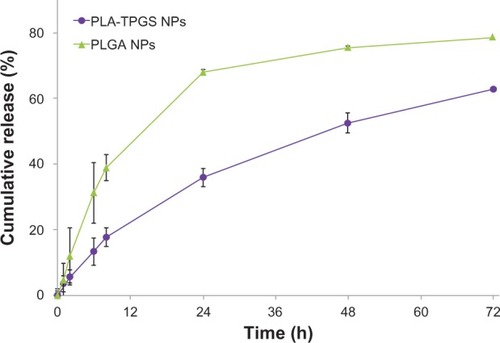
Table 8 Pharmacokinetic parameters in rats after iv injecting of Taxotere and DTX-loaded nanoparticles at a dose of 10 mg DTX/kg (n = 4)
Figure 7 Pharmacokinetic behavior after intravenous injection at a dose of docetaxel (DTX) 10 mg/kg to Sprague Dawley rats of Taxotere and DTX-loaded poly(lactic-co-glycolic acid) (PLGA) and poly(lactide)-D-α-tocopheryl polyethylene glycol 1000 succinate (PLA-TPGS) nanoparticles (n = 4).
Abbreviation: NPs, nanoparticles.
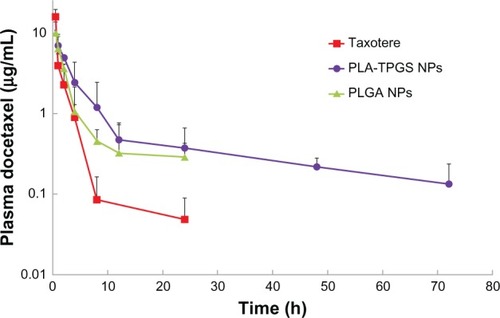
Figure 8 (A and B) Biodistribution of docetaxel-loaded nanoparticles. (A) In vivo images of H22 tumor-bearing mice after intravenous injection (dashed circles indicate tumors). (B) Ex vivo images of tumors and organs of H22-bearing mice killed at 30 hours.
Abbreviations: PLA-TPGS, poly(lactide)-D-α-tocopheryl polyethylene glycol 1000 succinate; PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles; h, hour.
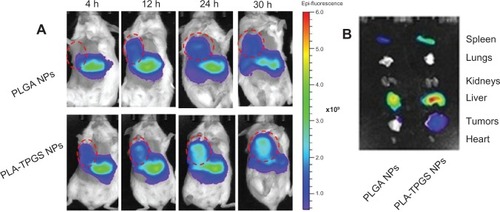
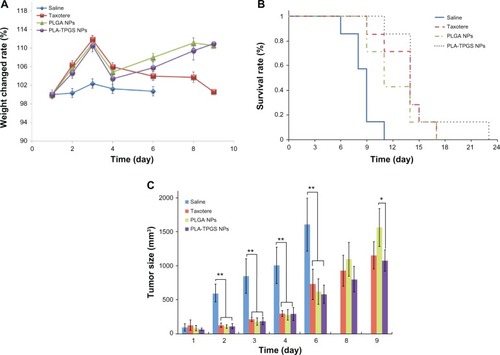
Figure 9 (A–C) In vivo antitumor efficiency of Taxotere and docetaxel-loaded poly(lactic-co-glycolic acid) (PLGA) and poly(lactide)-D-α-tocopheryl polyethylene glycol 1000 succinate (PLA-TPGS) nanoparticles (n = 7). (A) Weight changes of tumor-bearing mice; (B) survival rate of H22-bearing mice; (C) relative tumor-growth ratio (*P < 0.05, **P < 0.01).
Abbreviation: NPs, nanoparticles.