Figures & data
Figure 1 Transmission electron microscopy images of multiwalled (A) oxidized, (B) heparin, and (C) polyglycolic acid (PGA) carbon nanotubes at low magnification (mag), and (D) multiwalled PGA carbon nanotubes at high magnification. The arrows in (D) denote the formation of polymeric micelles on the multiwalled carbon nanotubes (MWCNTs).
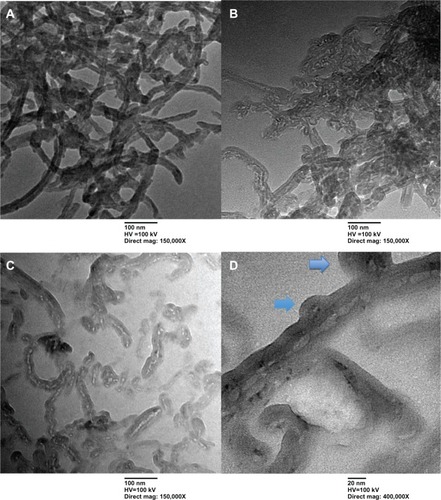
Figure 2 Scanning electron microscopy images of multiwalled (A) oxidized, (B) heparin, and (C) polyglycolic acid carbon nanotubes. The blue circles in (D) denote the formation of polymeric micelles on multiwalled carbon nanotubes (MWCNTs).
Abbreviations: NTUST, National Taiwan University of Science and Technology; SEI, second electron imaging; WD, working distance.
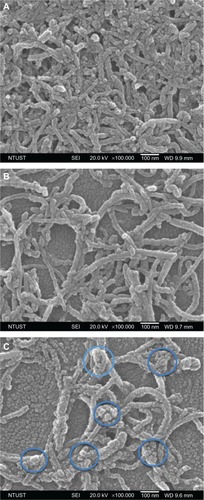
Figure 3 Raman spectra of multiwalled (MW) oxidized (COOH), MW heparin (Hep), and MW polyglycolic acid (PGA) carbon nanotubes.
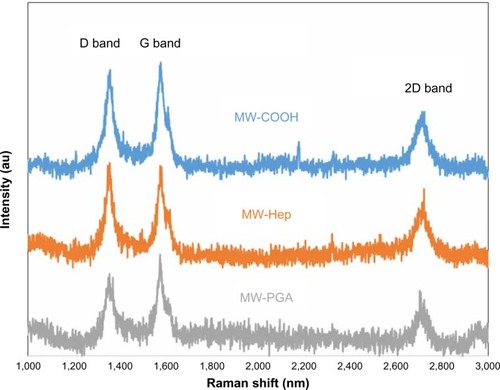
Figure 4 Particle size and distribution of multiwalled (A) oxidized, (B) heparin, and (C) polyglycolic acid carbon nanotubes analyzed by dynamic light scattering.
Abbreviation: PDI, polydispersity index; Z-average, hydrodynamic diameter.
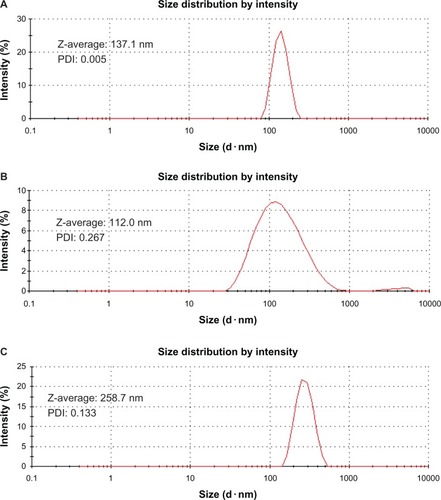
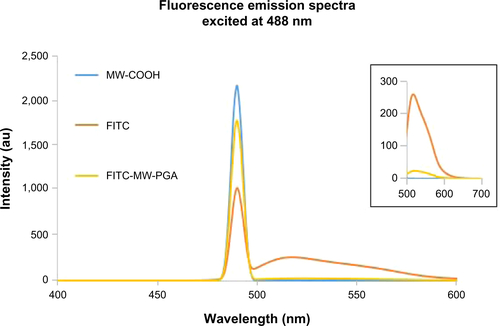
Figure 5 Plot of the fluorescence-intensity ratio of excimer/monomer as a function of multiwalled (MW) oxidized (COOH), MW heparin, and MW polyglycolic acid (PGA) carbon nanotube concentration.
Note: I452/I380; the ration of fluorescence intensity of pyrene at 452 nm to 380 nm.
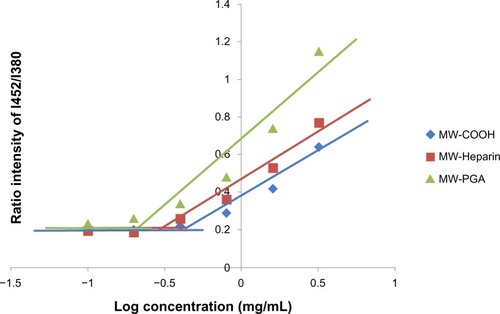
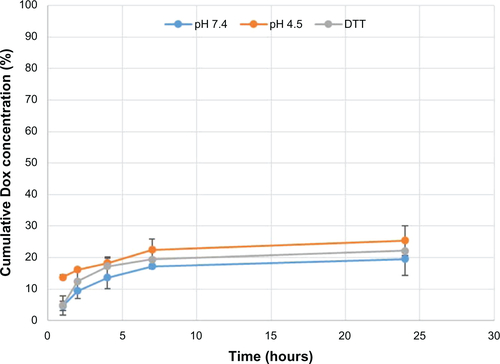
Table 1 Doxorubicin (Dox)-loading capacity of multiwalled oxidized (COOH), multiwalled heparin, and multiwalled polyglycolic acid (PGA) carbon nanotubes with a fixed weight of 0.162 mg
Figure 6 Zeta potentials of multiwalled (MW) oxidized (COOH), MW heparin (Hep), MW polyglycolic acid (PGA), doxorubicin (Dox)-loaded MW COOH, Dox-loaded MW Hep, and Dox-loaded MW PGA carbon nanotubes.
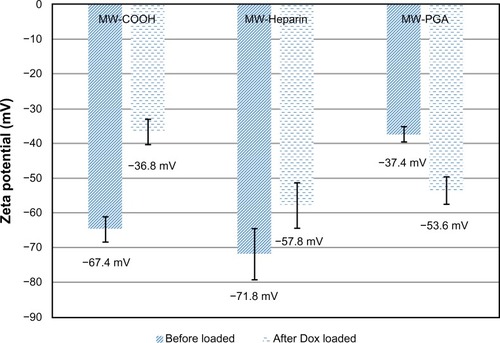
Figure 7 Drug-loading mechanism for the multiwalled polyglycolic acid (PGA) carbon nanotube (CNT) system and doxorubicin.
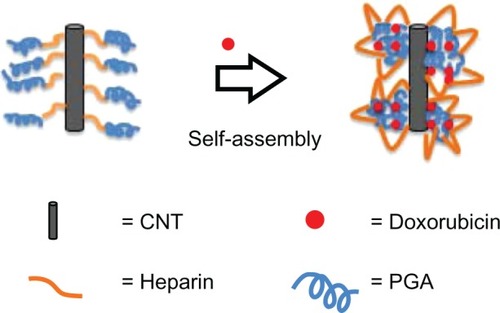
Figure 8 Cytotoxicity of multiwalled (MW) oxidized (COOH), MW heparin (Hep), MW polyglycolic acid (PGA) carbon nanotubes after incubation with HeLa cells for 24 hours at 37°C.
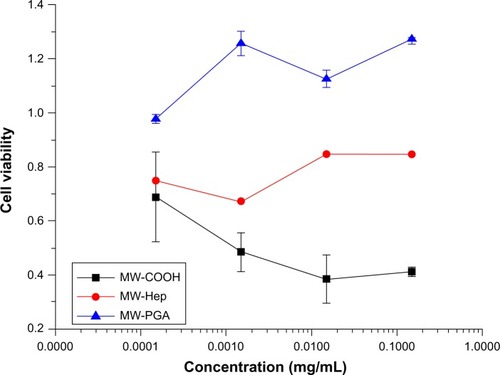
Figure 9 Confocal microscopy images of (A) free doxorubicin (Dox) and (B) multiwalled (MW) polyglycolic acid (PGA)-Dox carbon nanotubes incubated with HeLa cells for 2 hours. Red fluorescence means Dox and green fluorescence means PGA.
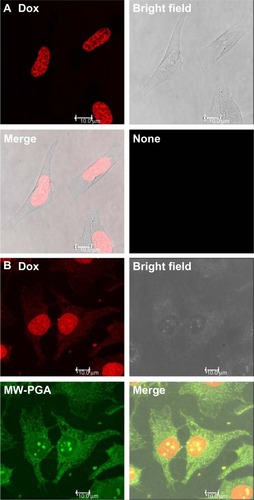
Figure 10 The cytotoxicity of free doxorubicin (Dox) and Dox-loaded multiwalled (MW) polyglycolic acid (PGA) carbon nanotubes after incubation with HeLa cells for 24 hours at 37°C
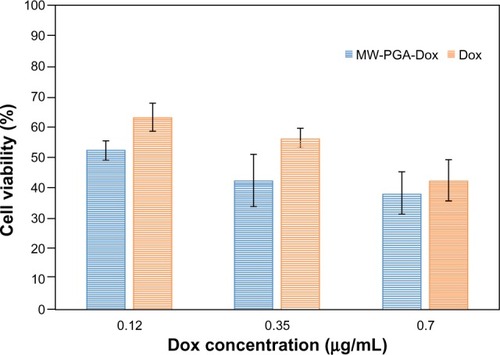
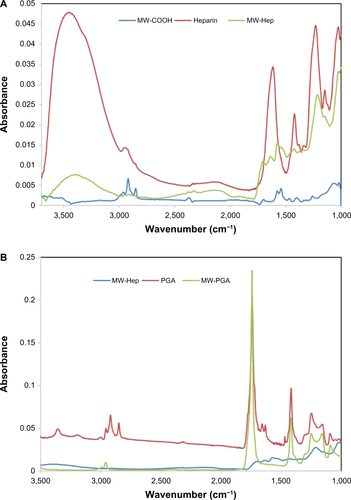
Figure S1 FTIR analysis of (A) multiwalled (MW) oxidized (COOH), heparin (Hep), MW Hep and (B) MW Hep, polyglycolic acid (PGA), and MW PGA carbon nanotubes.
Abbreviations: FTIR, Fourier-transform infrared.