Figures & data
Figure 1 Representation of the steric organization of a micelle (left), a liposome (center), and a lipid bilayer (right). Whereas liposomes are composed of a lipid bilayer, micelles are constructed of one lipid layer in which the apolar section turns inward and the polar heads interact with the environment. These two different organizations mean that the space enclosed in the micelles is much more limited to that available in liposomes.
Note: Adapted from Bitounis D, Fanciullino R, Iliadis A, Ciccolini J. Optimizing druggability through liposomal formulations: new approaches to an old concept. ISRN Pharm. 2012;2012:738432.Citation14
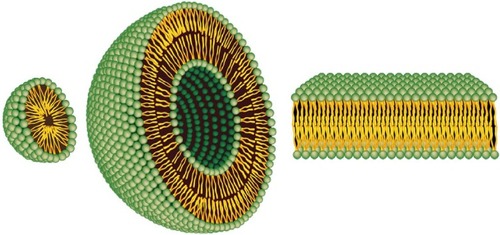
Figure 2 Targeting of nanomedicines by the enhanced permeability and retention (EPR) effect.
Notes: Differences between normal (A) and tumor (B) vessels are depicted. Tumor vessels contain large fenestrations between the endothelial cells: this structural characteristic allows the nanoparticles (NPs) to reach the matrix and the tumor cells by the EPR effect. Conversely, normal tissue contains tightly joined endothelial cells: this prevents the diffusion of NPs outside the blood vessels.
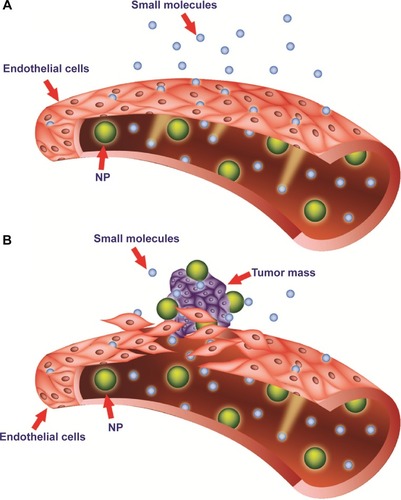
Figure 3 Liposome–cell interaction.
Notes: Liposomes loaded with a drug interact with the cell, binding to the surface through receptors (A). Absorption onto the plasma membrane can also occur by electrostatic interactions (B). The delivery of the cargo into the cell cytoplasm can take place through different modes. Lipid nanocarriers fuse with the plasma membrane and discharge drugs into the cell (C). After the interaction with the cell, the structure of the liposome bilayer can be affected and the cargo is released (D). Exchange of carrier-lipid components with the cell membrane can also occur (E). Liposomes internalized by endocytosis (F) can have different fates depending on physicochemical characteristics. Endosomes fuse with lysosomes (G): in this case, the low pH induces the degradation of the liposome membrane and the drug is released. Endosomes follow another route (H): liposomes release their cargo after fusion or the destabilization of the endocytic vesicle.
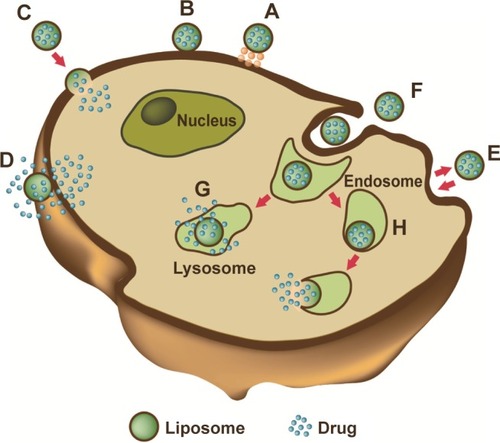
Figure 4 Replica of a cryofixed and cross-fractured human glioblastoma cell, interacting with dimyristoyl-sn-glycero-phosphatidylcholine liposomes. Liposomes appear mainly clustered on a pole of the cell (arrows). The fracture passes through the cytoplasm, and reveals the nucleus (asterisk) and the cytoplasmic organelles (arrowhead).
Figure 5 Replica of a cryofixed and cross-fractured human glioblastoma cell, interacting with dimyristoyl-sn-glycero-phosphatidylcholine liposomes. Liposomes (arrows) appear in the extracellular space and the cytoplasm of the cell. Some of them are interacting with cytoplasmic organelles (arrowhead).
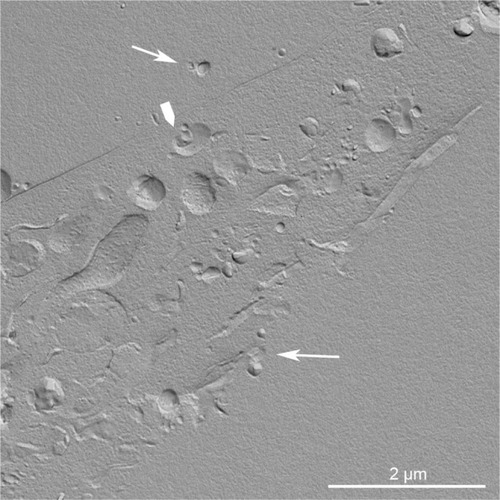

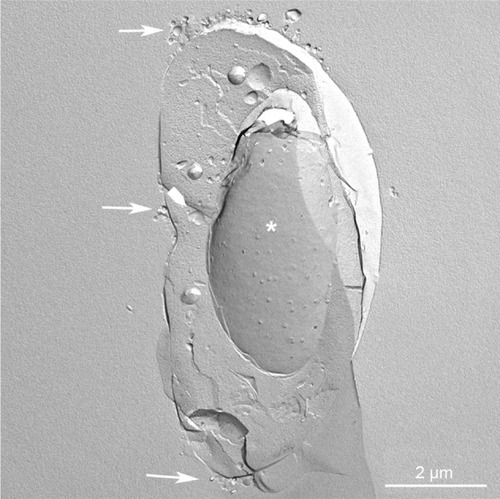
Figure 5 Replica of a cryofixed and cross-fractured human glioblastoma cell, interacting with dimyristoyl-sn-glycero-phosphatidylcholine liposomes. Liposomes (arrows) appear in the extracellular space and the cytoplasm of the cell. Some of them are interacting with cytoplasmic organelles (arrowhead).

Table 1 Liposomes on market or in clinical trials
Table 2 Liposomes on market or in clinical trials
