Figures & data
Figure 1 Preparation of silica NPs.
Notes: (A) Conversion of orthosilicic acid to silicon dioxide. (B) Manufacturing process for silica by neutralization reaction. The silica NP medium was prepared by incubating silica in culture medium for 48 hours and then by filtering through a 0.2 μm filter.
Abbreviations: DW, distilled water; PBS, phosphate-buffered saline; NPs, nanoparticles.
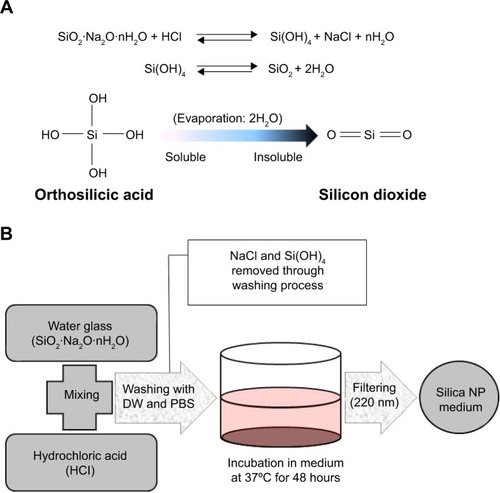
Figure 2 Transmission electron microscopy of different sized particles and observation of treated cells.
Notes: (A) NP and (B) MP media were observed by transmission electron microscopy. Adipose tissue-derived stem cells were incubated in each type of medium for 24 hours and then observed by transmission electron microscopy. Representative images are shown for (C) untreated cells, (D) silica MP-treated cells, and (E, F) silica NP-treated cells. Black arrows indicate particles and the triangle indicates a vesicle.
Abbreviations: NP, nanoparticle; MP, microparticle.
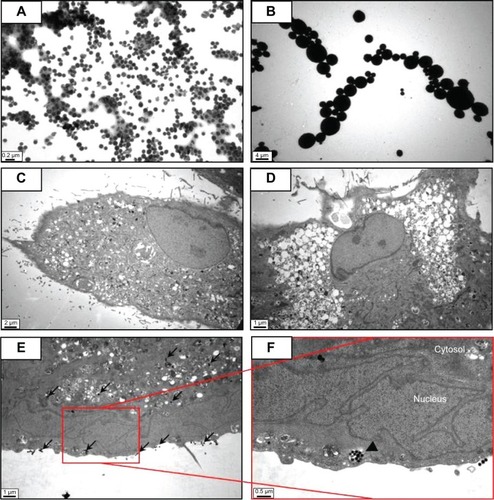
Figure 3 Proliferation and apoptosis assays in ADSCs treated with silica particles.
Notes: (A) Proliferation assay was performed by measuring DNA content. Total DNA (ng/μL) was increased in silica NPs with 1% FBS group compared with NP and MP media groups (*P<0.001). Experiments were performed in at least triplicate. The data are representative of three independent experiments. (B) Induction of apoptosis in human ADSCs treated with silica NP medium or silica MP medium was detected by Annexin V assay. ADSCs were treated with silica NPs or silica MPs for 6 and 72 hours after the cells were incubated in FBS-free medium for 24 hours. (C) DAPI staining of cells incubated in each type of medium for 24 hours. White arrows indicate DNA fragmentation in silica MPs.
Abbreviations: ADSCs, adipose tissue-derived stem cells; DAPI, 4,6-diamidino-2-phenyllindile; FBS, fetal bovine serum; NPs, nanoparticles; MPs, microparticles; SiNPs, silicon nanoparticles; SiMPs, silicon microparticles; PI, propidium iodide; FITC, fluorescein isothiocyanate.
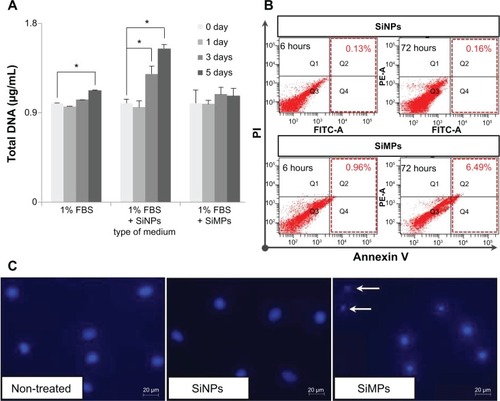
Figure 4 Activation of mitogen-activated protein kinases by differently sized silica particles.
Notes: (A) Protein extracts from the cells incubated in each type of medium for 10, 20, 30 and 60 minutes after starving in FBS-free medium for 24 hours were immunoblotted. (B, C) Quantitative analysis of ERK1/2 and p38 phosphorylation level by densitometric analysis. Three independent experiments were performed (*P<0.01, **P<0.001). (D) ERK1/2 activation by silica NPs was evaluated by treatment with PD98059, a MEK-ERK inhibitor. Protein was extracted from cells pretreated with PD98059 for 30 minutes, followed by treatment with silica NPs for 10 minutes. Quantitative analysis of three independent experiments by densitometric analysis was performed. ERK1/2 phosphorylation by basic fibroblast growth factor and silica NPs was prevented by pretreatment with PD98059 (*P<0.01, **P<0.001). (E) A proliferation assay was performed by measuring DNA content in cells pretreated with PD98059 followed by treatment with silica NPs. Total DNA (ng/μL) was increased in the silica NPs with 1% FBS group, whereas the pretreated group showed reduced proliferation. Experiments were performed in triplicate at least.
Abbreviations: ERK, extracellular signal-related kinase; JNK, Janus kinase; NPs, nanoparticles; MPs, microparticles; bFGF, basic fibroblast growth factor; p-, phosphorylated; MEK, mitogen-activated protein kinase kinase; FBS, fetal bovine serum.
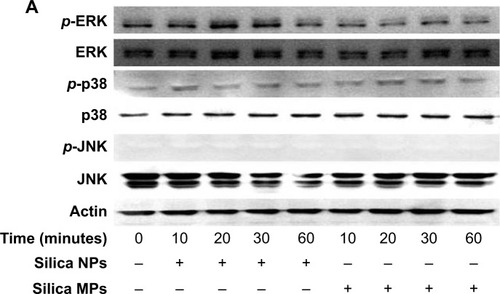
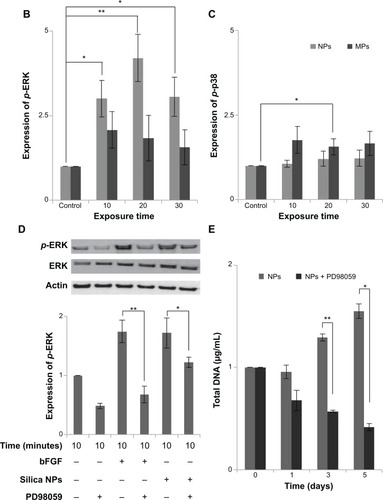
Figure 5 Proposed signaling pathways affected by silica NPs and silica MPs.
Abbreviations: NPs, nanoparticles; MPs, microparticles; ERK, extracellular signal-related kinase; MEK, mitogen-activated protein kinase kinase.
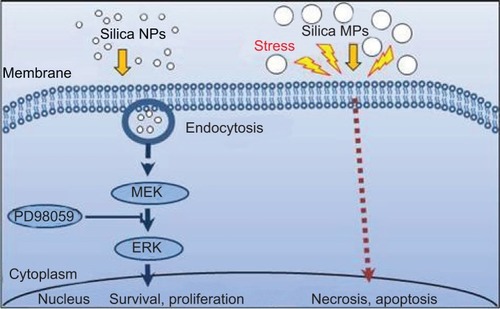
Figure S1 Analysis of purified silicon dioxide (silica).
Notes: (A) Purity (99.81%) of prepared silica gel was determined by X-ray fluorescence analysis. (B) Standard curve indicates that the amount of silicon is proportional to the amount of sodium silicate that was reacted with hydrochloric acid. The amount of silicon was analyzed by an inductively coupled plasma method.
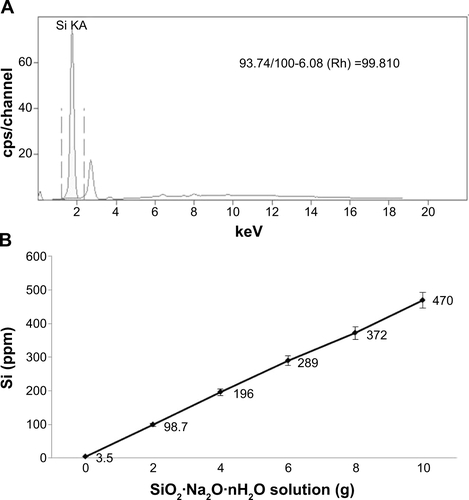
Figure S2 Particle distribution of silica NPs and silica MPs.
Notes: (A) Silica NP distribution according to size, ie, <50 nm (1.6%), 50–80 nm (21%), 80–120 nm (68%), and 120–150 nm (8.9%). (B) Silica MP distribution according to size, ie, <2 μm (5%), 2–4 μm (78%), and >4 μm (17%) (*P<0.01).
Abbreviations: NPs, nanoparticles; MPs, microparticles.
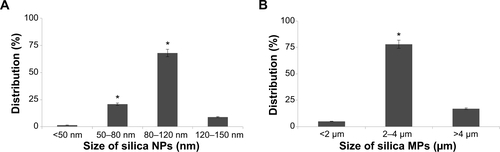
Figure S3 Transmission electron microscopic images of cells treated with silica nanoparticles.
Notes: (A) and (B) show cell membrane that vesicles entered to cytosol by endocytosis and triangle arrow indicates endocytosis of nanoparticles.
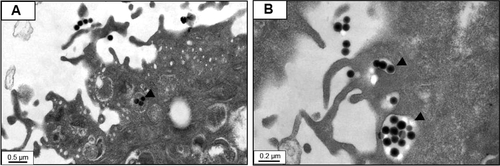
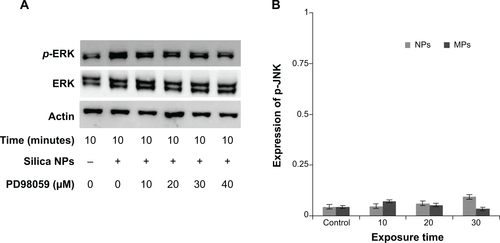
Figure S4 ERK and JNK Phosphorylation of hADSCs with silica NPs and MPs.
Notes: (A) Phosphorylation of ERK induced by silica NPs is inhibited by PD98059 in a dose-dependent manner. (B) Quantitative analysis of JNK phosphorylation level of ADSCs by densitometric analysis according to silica NPs and silica MPs. Reproduced with permission from Kim KJ, Jeon YJ, Lee JH, et al. The Effect of Silicon Ion on Proliferation and Osteogenic Differentiation of Human ADSCs. Korea Tissue Engineering and Regenerative medicine. 2010;7(2):171–177.Citation1
Abbreviations: NPs, nanoparticles; MPs, microparticles; ERK, extracellular signal-related kinase; JNK, Jun amino-terminal kinases; PD98059, MEK-ERK inhibitor.