Figures & data
Figure 1 Expression and characterization of H53.
Notes: (A) The HA ectodomain (nucleotides 1–1,723) was amplified from pHW500 and modified by addition of linker sequences, a GCN4pII trimerization domain, and His-tag sequences at the 3′ end. (B) SDS-PAGE and Blue Native gel electrophoresis confirmed the expression of the H5 monomer and trimeric forms. (C) A neutralization inhibition assay demonstrated that the immunogenicity of trimeric H5 was significantly greater than that of monomeric forms, requiring a lower concentration of protein to inhibit 50% of the neutralizing sera (IC50). Error bars represent the standard error of the mean. *Indicates statistical significance. P≤0.0243.
Abbreviations: HA, hemagglutinin; H5, H5 hemagglutinin monomer; H53, H5 hemagglutinin trimer; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; IC50, inhibitory concentration 50%.
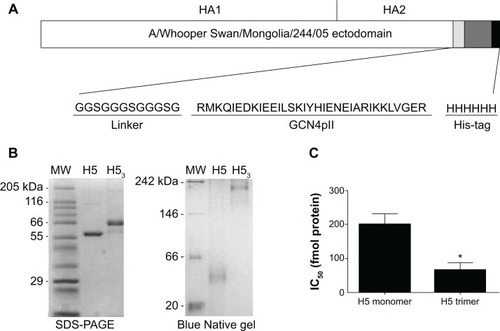
Table 1 H53 vaccine formulations
Figure 2 Immunogenicity of soluble H53 antigen.
Notes: (A) Mice were immunized subcutaneously or intranasally with 10 μg soluble H53 on days 0, 21, and 42. Sera collected at days 21, 42, and 63 after primary immunization were tested for neutralizing antibody. Neutralizing antibody titers were not detected until after the second immunization; however, titer generated by days 42 and 63 were robust. (B) The neutralizing antibody titers demonstrated cross-clade protection at 63 days with both immunization routes. Error bars represent the standard error of the mean.
Abbreviations: H53, H5 hemagglutinin trimer; ID50, sera dilution that inhibits 50% of pseudovirus infectivity.
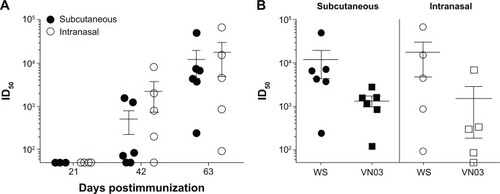
Figure 3 The neutralizing antibody responses to H53-polyanhydride nanovaccines were examined 42 and 63 days post-primary immunization.
Notes: Single-dose vaccination regimens utilizing poly I:C encapsulated with H53 demonstrated neutralization titers similar to a MPLA-adjuvanted control at day 42, with most of the remaining formulations reaching equivalency at day 63. The use of a prime/boost/boost immunization regimen enhanced the production of neutralizing antibodies and reduced mouse-to-mouse variability. Error bars represent the standard error of the mean. Different letters indicate statistical significance among treatments of the same regimen (ie, single dose or prime/boost/boost) at each time point. *Indicates statistical significance between the different regimens for each treatment. No statistical significance was observed when comparing between the various prime/boost/boost formulations. P≤0.0434.
Abbreviations: H53, H5 hemagglutinin trimer; MPLA, monophosphoryl lipid A; sH53, soluble H5 hemagglutinin trimer; ID50, sera dilution that inhibits 50% of pseudovirus infectivity; poly I:C, polyinosinic-polycytidylic acid.
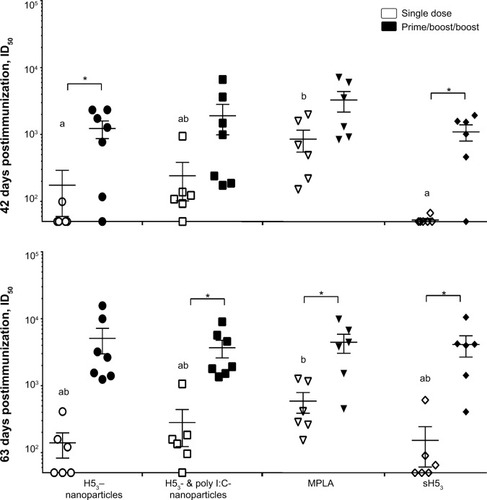
Figure 4 Enhanced CD4+ T cell memory with poly I:C polyanhydride nanovaccine.
Notes: Draining lymph nodes were harvested from prime/boost/boost immunized mice 63 days after the primary immunization. Ex vivo antigen stimulation and CFSE-labeling demonstrated enhanced CD4+ T cell proliferation of mice immunized with poly I:C nanovaccines. Error bars represent the standard error of the mean. Different letters indicate statistical significance among treatments. P≤0.0147.
Abbreviations: CFSE, 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester; MPLA, monophosphoryl lipid A; sH53, soluble H5 hemagglutinin trimer; poly I:C, polyinosinic-polycytidylic acid..
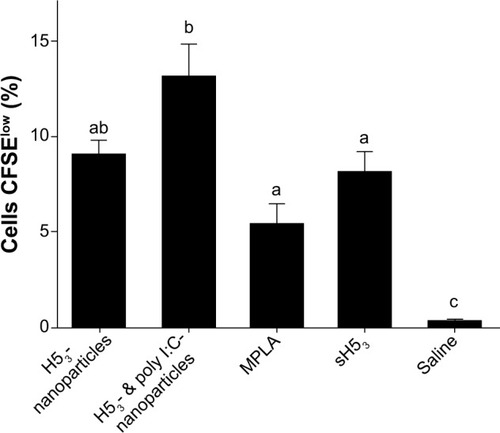
Figure 5 Mice were protected upon challenge with low pathogenic VNH5N1-PR8/CDC-RG.
Notes: (A) Mice were challenged with a reverse genetics-derived influenza virus, VNH5N1-PR8/CDC-RG, containing the HA and NA genes of A/Vietnam/1203/04 (H5N1) virus in the genetic background of the high-growth master strain A/Puerto Rico/8/34 (H1N1). Body weight was observed for 2 weeks postinfection. All vaccinated mice maintained or gained weight similar to healthy naïve, no challenge mice. (B) Viral load in lung homogenates collected at 3 days post-challenge, (C) which correlated with the pre-challenge neutralizing antibody titers. Different letters indicate statistical significant among treatments.
Abbreviations: H53, H5 hemagglutinin trimer; HA, hemagglutinin; ID50, sera dilution that inhibits 50% of pseudovirus infectivity; NA, neuraminidase; sH53, soluble H5 hemagglutinin trimer; TCID50, tissue culture infectious dose 50%.
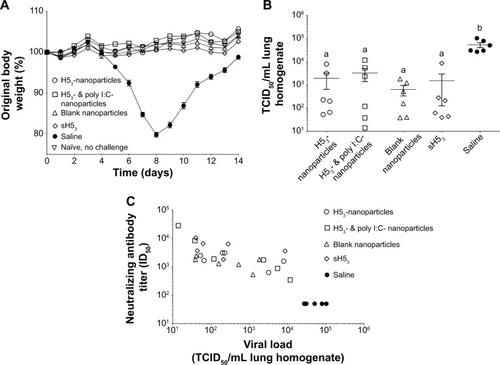
Figure 6 H53-nanoparticle responses most similar to healthy naïve, no challenge mice.
Notes: (A) Principal component analysis was performed utilizing neutralizing antibody titer, viral load, and inflammatory cytokine measurements, with each data point representing one mouse. All vaccination formulations were found to be very different from saline-administered mice, as evidenced by the small and high PC1 values, respectively. (B) PCA displaying average values after the exclusion of saline-administered mice and neutralizing antibody titers. The H53-nanoparticle formulation induced responses very similar to healthy, noninfected mice.
Abbreviations: H53, H5 hemagglutinin trimer; PC, principal components; PCA, principal component analysis.
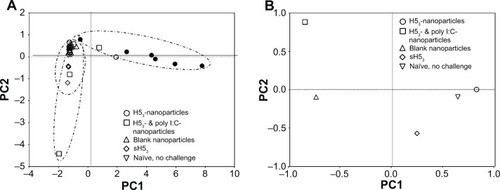
Figure S1 Prime/boost/boost immunized mice induced protective neutralizing antibody titers.
Notes: Mice immunized with the various formulations were challenged with a low pathogenic influenza virus 63 days after the initial vaccination. One week before the challenge, all vaccine formulations induced high neutralizing antibody titers consistent with previous work and were suggested to be protective. Error bars represent the standard error of the mean. Different letters indicate statistical significance among treatments. P≤0.0001.
Abbreviations: ID50, sera dilution that inhibits 50% of pseudovirus infectivity; H53, H5 hemagglutinin trimer; sH53, soluble H5 hemagglutinin trimer.
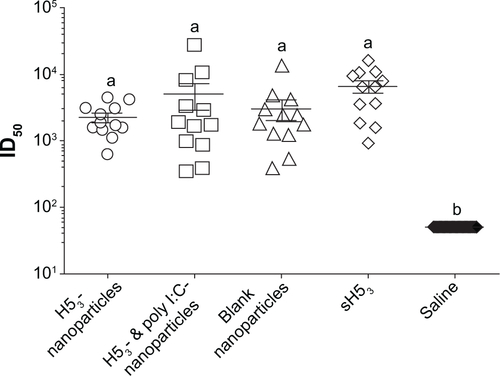
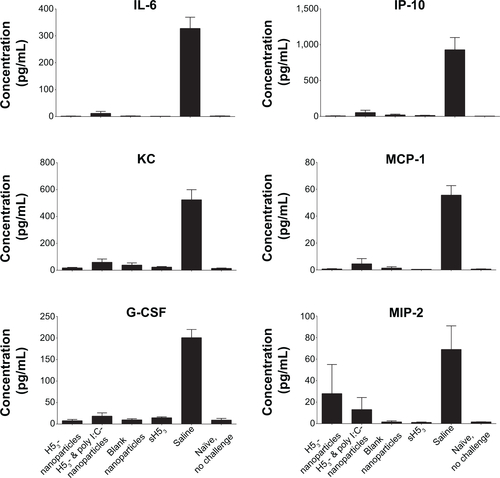
Figure S2 Low levels of inflammatory cytokines were present in immunized mice postchallenge.
Notes: Saline-administered mouse lungs had significant increases in inflammatory cytokines 3 days postchallenge. In contrast, the lungs of H53-immunized mice had low levels of inflammatory cytokines similar to healthy, noninfected mice.
Abbreviations: H53, H5 hemagglutinin trimer; sH53, soluble H5 hemagglutinin trimer.