Figures & data
Figure 1 Chromatic properties of culture supernatant of Brevibacterium frigoritolerans DC2.
Notes: Culture supernatant of B. frigoritolerans DC2 after incubation with AgNO3 (silver nitrate) (1 mM) (A) and control with medium and AgNO3 (1 mM) after incubation period (B). UV-Vis spectra of culture supernatant of B. frigoritolerans DC2 treated with 1 mM AgNO3 (C).
Abbreviations: OD, optical density; UV-Vis, ultraviolet-visible.
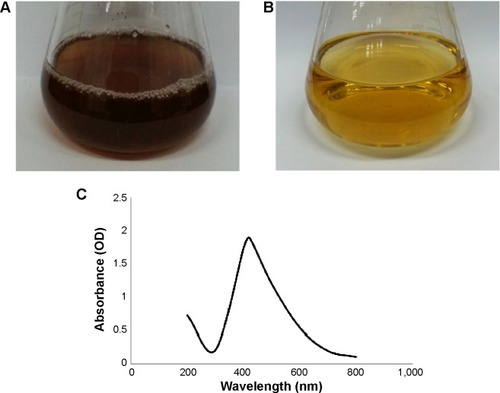
Figure 2 Properties of silver nanoparticles synthesized by Brevibacterium frigoritolerans DC2.
Notes: Transmission electron micrograph of silver nanoparticles synthesized by B. frigoritolerans DC2 (A and B). Particles size distribution of silver nanoparticles according to volume (C), number (D), and intensity (E).
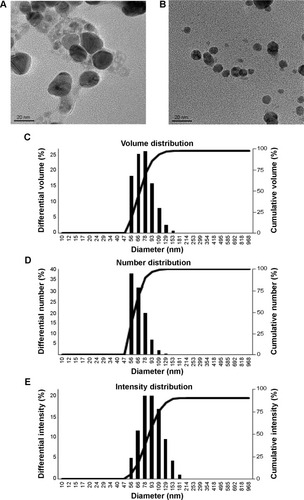
Figure 3 Silver nanoparticles examined under a variety of conditions.
Notes: Energy dispersive X-ray spectroscopy of the whole scan area showing major peak of silver nanoparticles at 3 keV (A); X-ray diffraction patterns of silver nanoparticles obtained by Brevibacterium frigoritolerans DC2 (B); transmission electron micrograph of silver nanoparticle pellet solution (C); silver nanoparticles, green (D).
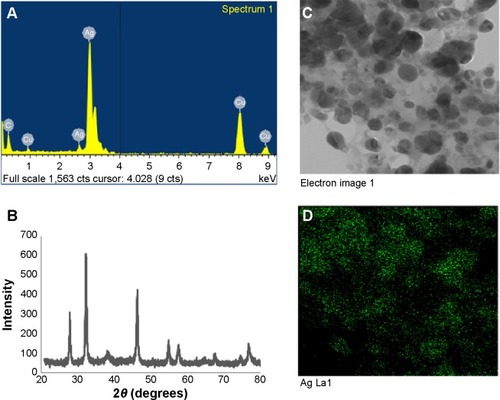
Table 1 Diameters of zones of inhibition (mm) for 50 μL of reaction mixture containing silver nanoparticles after incubation with various microorganisms
Figure 4 Zones of inhibition for various pathogens.
Notes: Zones of inhibition of 50 μL of reaction mixture containing silver nanoparticles and 50 μL of 1mM AgNO3 (silver nitrate) against the pathogenic strains Candida albicans (A), Vibrio parahaemolyticus (B), Bacillus anthracis (C), Bacillus cereus (D), Salmonella enterica (E), and Escherichia coli (F).
Abbreviation: AgNPs, silver nanoparticles.
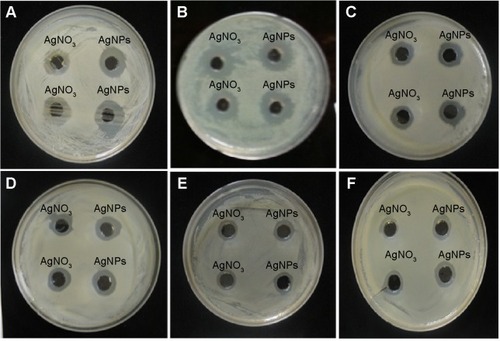
Figure 5 Effects of partially purified silver nanoparticle pellet solution on various pathogens.
Notes: Zones of inhibition of partially purified silver nanoparticle pellet solution and silver nitrate (AgNO3) (both at same concentration, 30 μL [100 mg/L]) against Salmonella enterica (A), Escherichia coli (B), Vibrio parahaemolyticus (C), Bacillus anthracis (D), and Bacillus cereus (E). Zones of inhibition of standard antibiotic discs against pathogenic bacteria Salmonella enterica (F), Escherichia coli (G), Vibrio parahaemolyticus (H), Bacillus anthracis (I), and Bacillus cereus (J). Zones of inhibition of standard antibiotic discs with silver nanoparticles against Salmonella enterica (K), Escherichia coli (L), Vibrio parahaemolyticus (M), Bacillus anthracis (N), and Bacillus cereus (O). Zones of inhibition of silver nanoparticles and silver nitrate against Candida albicans (P), cycloheximide (Q-7), Cycloheximide with AgNPs (Q-8,9). For (A–Q), the numbers, the corresponding antibiotics, and the corresponding concentrations of antibiotics are as follows: 1) lincomycin (MY15), 15 μg/disc; 2) oleandomycin (OL15), 15 μg/disc; 3) novobiocin (NV30), 30 μg/disc; 4) vancomycin (VA30), 30 μg/disc; 5) penicillin G (P10), 10 μg/disc; 6) rifampicin (RD5) 5 μg/disc; 7) cycloheximide, 10 μg/disc; 8) cycloheximide (10 μg/disc) with silver nanoparticles; and 9) cycloheximide (10 μg/disc) with silver nanoparticles.
Abbreviation: AgNPs, silver nanoparticles.
![Figure 5 Effects of partially purified silver nanoparticle pellet solution on various pathogens.Notes: Zones of inhibition of partially purified silver nanoparticle pellet solution and silver nitrate (AgNO3) (both at same concentration, 30 μL [100 mg/L]) against Salmonella enterica (A), Escherichia coli (B), Vibrio parahaemolyticus (C), Bacillus anthracis (D), and Bacillus cereus (E). Zones of inhibition of standard antibiotic discs against pathogenic bacteria Salmonella enterica (F), Escherichia coli (G), Vibrio parahaemolyticus (H), Bacillus anthracis (I), and Bacillus cereus (J). Zones of inhibition of standard antibiotic discs with silver nanoparticles against Salmonella enterica (K), Escherichia coli (L), Vibrio parahaemolyticus (M), Bacillus anthracis (N), and Bacillus cereus (O). Zones of inhibition of silver nanoparticles and silver nitrate against Candida albicans (P), cycloheximide (Q-7), Cycloheximide with AgNPs (Q-8,9). For (A–Q), the numbers, the corresponding antibiotics, and the corresponding concentrations of antibiotics are as follows: 1) lincomycin (MY15), 15 μg/disc; 2) oleandomycin (OL15), 15 μg/disc; 3) novobiocin (NV30), 30 μg/disc; 4) vancomycin (VA30), 30 μg/disc; 5) penicillin G (P10), 10 μg/disc; 6) rifampicin (RD5) 5 μg/disc; 7) cycloheximide, 10 μg/disc; 8) cycloheximide (10 μg/disc) with silver nanoparticles; and 9) cycloheximide (10 μg/disc) with silver nanoparticles.Abbreviation: AgNPs, silver nanoparticles.](/cms/asset/bb7d9c9a-7ad5-4110-9ed2-a4d6b0f835ae/dijn_a_72313_f0005_c.jpg)
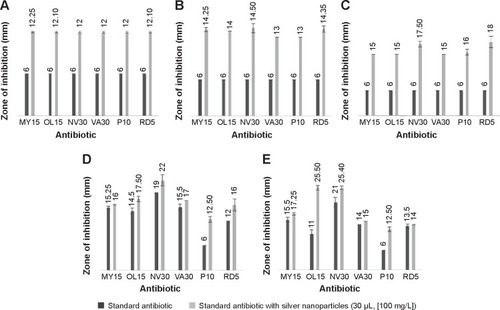
Figure 6 Graphical representation of comparative study between antibiotics and antibiotics with AgNPs.
Notes: Salmonella enterica (A), Escherichia coli (B), Vibrio parahaemolyticus (C), Bacillus anthracis (D), and Bacillus cereus (E), respectively. Results interpreting the antimicrobial activity of commercial antibiotics alone with that of antibiotics combined with silver nanoparticles.