Figures & data
Figure 1 Scanning electron micrograph of amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound.
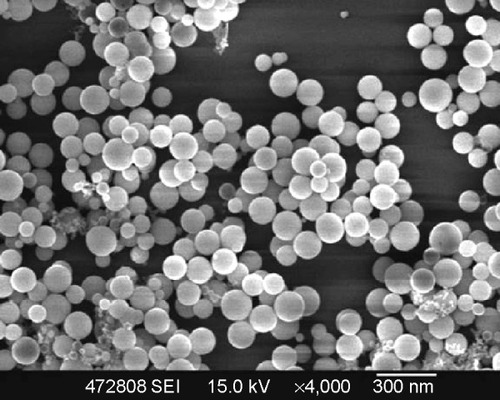
Figure 2 ACC/CaIP6 nanoparticle siRNA-binding efficiency and cytotoxicity in MCF-7 cells.
Notes: (A) Gel electrophoresis was used to assess ACC/CaIP6 nanoparticles and siRNA binding at different mass ratios. (B) Cytotoxicity was measured by the percentage of viable MCF-7 cells present after ACC/CaIP6 treatment, using the MTT assay 48 hours post–transfection. These percentages were calculated in relation to those of untreated controls. The data show the mean ± standard deviation of three independent experiments (*P<0.05 by one-way analysis of variance).
Abbreviations: ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; siNC, control small interfering RNA; lipo, Lipofectamine 2000; siRNA, small interfering RNA.
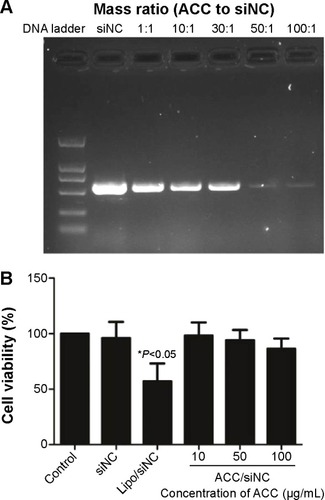
Figure 3 ACC/CaIP6/siRNA transfection efficiency in MCF-7 cells.
Notes: (A) Inverted fluorescence microscopy images showing FAM-siRNA fluorescence 6 hours after transfection. (B) Representative histograms and percentages (mean ± standard deviation) of fluorescent cells as measured by flow cytometry. The data show the mean ± standard deviation of three independent experiments.
Abbreviations: ACC, amorphous calcium carbonate; ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; FAM-siAKT1, fluorescein-labeled AKT1-specific small interfering RNA; lipo, Lipofectamine 2000.
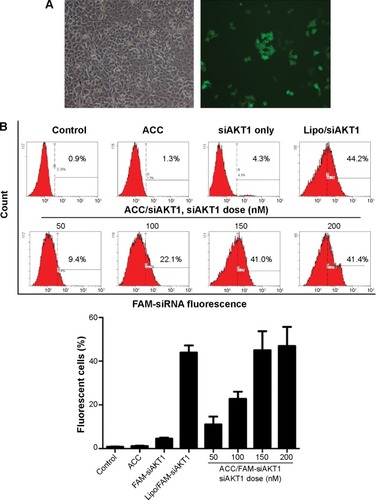
Figure 4 Intracellular siAKT1 distribution in MCF-7 cells.
Notes: FAM-siAKT1 were labeled with fluorescein (green). Cell nuclei were stained with DAPI (blue).
Abbreviations: FAM-siAKT1, fluorescein-labeled AKT1-specific small interfering RNA; DAPI, 4′,6-diamidino-2-phenylindole.
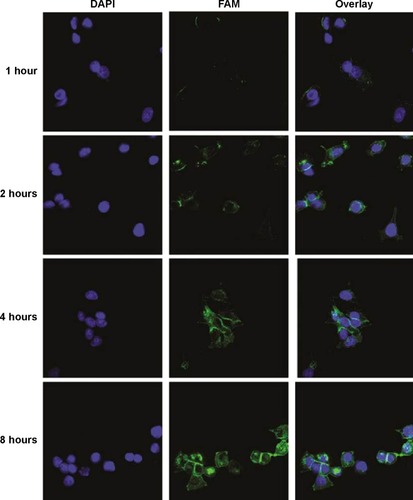
Figure 5 In vitro AKT1 silencing by ACC/CaIP6/siAKT1 transfection in MCF-7 cells.
Notes: (A) AKT1 mRNA and protein expression after transfecting MCF-7 cells with ACC/CaIP6/siAKT1 complexes was assessed by real-time polymerase chain reaction and Western blots, respectively. ***P<0.001 versus control. (B) The MTT assay was used to assess MCF-7 cell viability after transfecting cells with ACC/CaIP6 complexes containing different concentrations of siAKT1 (*P<0.05, ***P<0.001 versus control). (C) Flow cytometry was used to determine the cell cycles of MCF-7 cells 48 hours after ACC/CaIP6/siAKT1 transfection. (D) Apoptosis was measured using Annexin V-phycoerythrin/propidium iodide staining for MCF-7 cells treated with various ACC/CaIP6/siRNA complexes (one of three replicates is shown).
Abbreviations: ACC, amorphous cal cium carbonate; ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; siAKT1, small interfering AKT1; siNC, control small interfering RNA; OD, optical density; PI, propidium iodide.
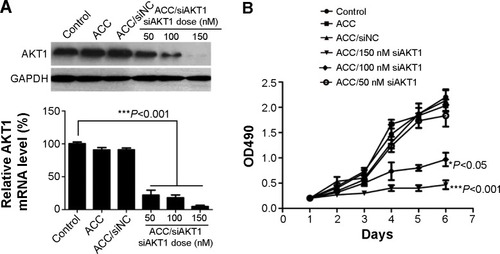
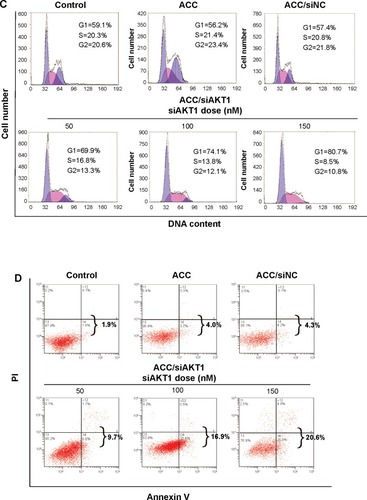
Figure 6 Tumor growth in vivo after intratumoral injections of ACC/CaIP6/siAKT1 nanoparticles.
Notes: (A) Representative tumors before and after injection. (B) ACC/CaIP6/siAKT1 (20 µg siAKT1/injection, 50:1 mass ratio) was locally injected into the tumors (100 mm3 in volume at 12 days). Tumor diameter was measured using calipers and tumor volume was calculated using the following formula: volume = W2 × L/2, where W is the width of the tumor and L is the tumor length. Results are presented as the mean ± standard deviation (n=7 per group; ***P<0.001 versus other groups). (C) Mean tumor weight (± standard deviation) after mice were sacrificed (n=7 per group; **P<0.01 versus other groups).
Abbreviations: ACC, amorphous calcium carbonate; ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; siAKT1, small interfering AKT1.
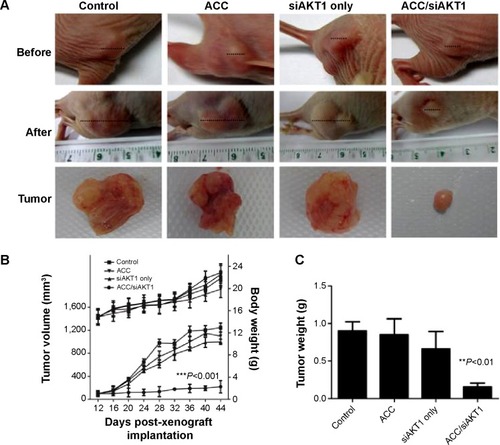
Figure S1 Intracellular distribution of ACC/CaIP6/FAM-siAKT1 in MCF-7 cells was analyzed by confocal laser scanning microscopy after incubation for 8 hours. Lysosomes were stained with Lyso Tracker Red, a percentage of the fluorescence of FAM-siAKT (green) did not overlap with the fluorescence of lysosomes (red), indicating successful escape of the FAM-siRNA from the lysosomes to the cytoplasm.
Abbreviations: ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; FAM-siAKT1, fluorescein-labeled AKT1-specific small interfering RNA.
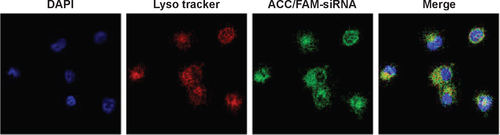
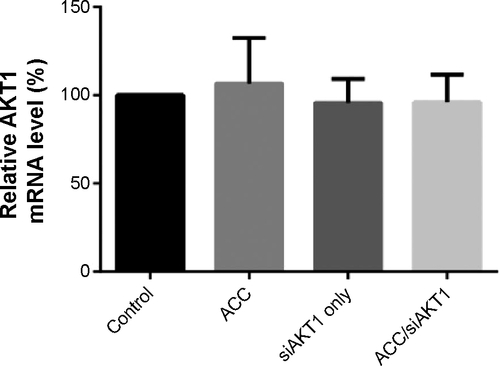
Figure S2 Real-time quantitative polymerase chain reaction analysis of the relative expression of AKT1 mRNA in liver samples intratumorally injected with ACC, siAKT1, and ACC/CaIP6/siAKT1 nanoparticles.
Abbreviations: ACC/CaIP6, amorphous calcium carbonate hybrid nanospheres functionalized with a Ca(II)-inositol hexakisphosphate compound; siAKT1, small interfering AKT1.