Figures & data
Table 1 Comparison of physicochemical and biological characters in CSC-Fe-bLf NCs and ACSC-Fe-bLf NCs
Figure 1 Formulation and physicochemical characterization of ceramic NCs.
Notes: (A) The ACSC-Fe-bLf NCs were found to have spherical morphology when observed under (B) transmission electron microscopy. Sphericity and size of ACSC-Fe-bLf NCs were reconfirmed by (C) scanning electron microscopy. (D) Size determination using dynamic light scattering spectrometry confirmed the size of 322±27.2 nm for ACSC-Fe-bLf NCs. (E) Controlled release of Fe-bLf from ACSC NCs as observed under pH 1.5, pH 2, and pH 8 in a range of time starting from 1 hour to 96 hours. (F) The physicochemical characterization of the NCs was studied using Fourier transform infrared spectroscopy analysis. (G) Thermogravimetric analysis of NCs represented the arrangement of different layers to protect the encapsulated protein.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; Cap, calcium phosphate; CSC, chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; NCs, nanocapsules/nanocarriers.
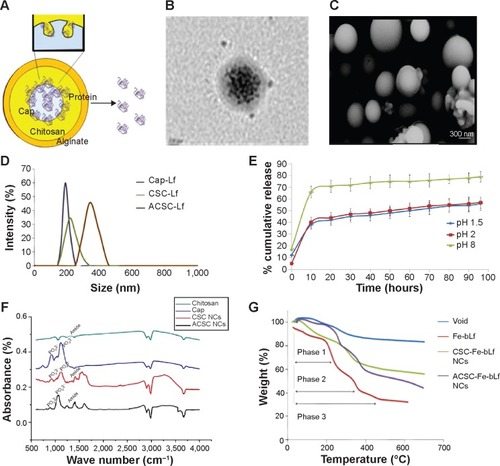
Figure 2 Stability and in vitro internalization efficacy of ceramic NCs.
Notes: (A) The differential scanning calorimetry curve showed modification in the melting point and the enthalpy of melting of NCs. (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis showed the stability of NC-encapsulated Fe-bLf at varying pH (2–8). (C) It was observed using dynamic light scattering that alginate layer was shed off from ACSC-Fe-bLf NCs at alkaline pH to expose the CSC-Fe-bLf NCs. (D) Confocal microscopy confirmed successful internalization of rhodamine-labeled NCs in 1 hour, which was found to increase with time. (E) Flow cytometric analysis shows an increase in the internalization of rhodamine-loaded CSC-Fe-bLf NCs compared to void CSC NCs. (F) NCs enter the cells mainly via clathrin-mediated endocytosis and energy-mediated pathways. (G) 3.3 mg/mL of Fe-bLf loaded in NCs induced a 130-fold reduction in the size of multicellular tumor spheroids at 72 hours and 96 hours. Data represent mean ± standard error of the mean. Experiments were repeated three times independently with similar results. *P<0.05; **P<0.005; ***P≤0.0005.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; CSC, chitosan-coated calcium phosphate; DAPI, 4′,6-diamidino-2-phenylindole; Fe-bLf, iron-saturated bovine lactoferrin; LRP, low-density lipoprotein receptor; m/w, molecular weight; NCs, nanocapsules/nanocarriers; TfR, transferrin receptor.
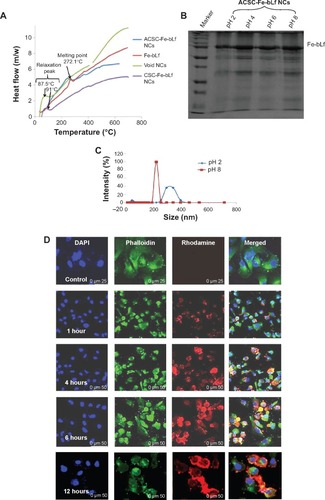
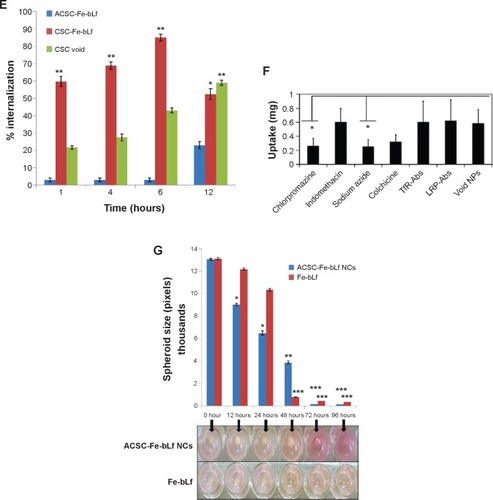
Figure 3 In vitro anticancer efficacy of Fe-bLf-encapsulating ceramic NCs.
Notes: (A) The IC50 values as determined using MTT at 10 hours for void NCs was 3.83 mg/mL, for ACSC-Fe-bLf it was 1.69 mg/mL, and for CSC-Fe-bLf it was 1.20 mg/mL. (B) The results from CyQUANT after 10 hours showed that CSC-Fe-bLf (42.8%) significantly lowered (P≤0.005) the cell proliferation when compared to void NCs (92.2%) and ACSC-Fe-bLf NCs (93%). (C) The IC50 values determined using MTT at 20 hours for void NCs was found to be 4.8 mg/mL, for ACSC-Fe-bLf NCs it was 1.62 mg/mL, and for CSC-Fe-bLf NCs it was 1.19 mg/mL. (D) The cell proliferation results from CyQUANT after 20 hours showed that CSC-Fe-bLf (15.3%) significantly (P≤0.005) lowered the cell proliferation when compared to ACSC-Fe-bLf NCs (63%) and void NCs (72.6%). Data represent mean ± standard error of the mean. Experiments were repeated three times independently with similar results **P≤0.005.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; CSC, chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; NCs, nanocapsules/nanocarriers.
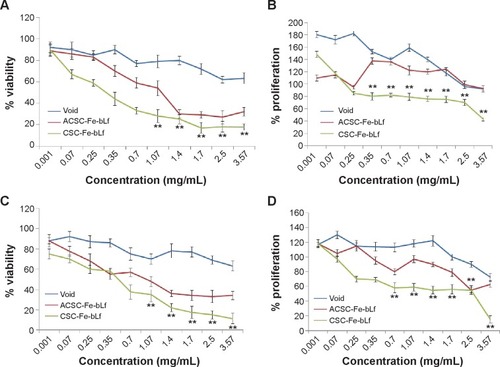
Figure 4 In vivo antitumor efficacy of ceramic NCs.
Notes: (A) Within 1 week after the injection of 107 cancer cells, tumors (~45.6 mm3) were developed in control diet–fed mice and were terminated by injecting Taxol (IP) when challenged for the second time, ~100.3 mm3 tumors were observed in 4 weeks. In neither of the cases (first challenge or the second challenge), significant tumors were developed in the treatment groups of mice. (B) Average body weight of the control group remained at 14.41 g, whereas treatment groups showed an increase in body weight up to 17.32 g. (C) ASCS-Fe-bLf NC diet showed 10-fold reduction in tumor volume from 55th day to 80th day, and on 85th day, the tumor was completely absent. Data represent mean ± standard error of the mean (n=5). *P<0.05.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; Dox, doxorubicin; Fe-bLf, iron-saturated bovine lactoferrin; IP, intraperitoneal; NCs, nanocapsules/nanocarriers.
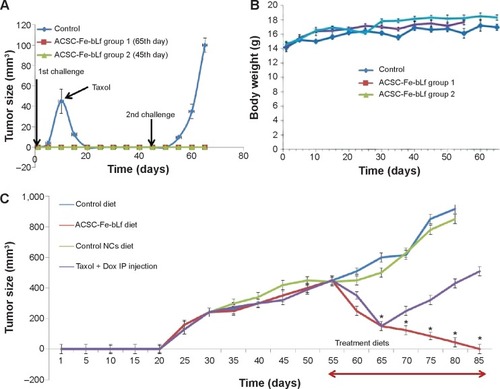
Figure 5 Histological analysis for cytotoxicity studies.
Notes: (A) Tissue pathology observation after the treatment is represented in the figure. Hematoxylin and eosin staining was carried out in order to observe the tissue integrity in the control and treatment groups. Five-micrometer-thin slices of intestine (TS), kidney (LS), and brain (LS) from control (a, c, and e) and treated (b, d, f) mice were stained with hematoxylin and eosin was used as a counterstain. Observations were made under 100× oil immersion objective. (B) Immunohistochemical localization of ACSC-Fe-bLf NCs using anti-lactoferrin antibody. Brain (LS), liver (LS), and intestine (TS) sections from control (a, c, e) and treated (b, d, f) groups are shown.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; LS, longitudinal section; NCs, nanocapsules/nanocarriers; TS, transverse section.
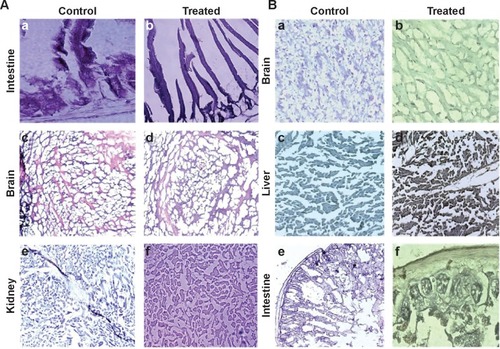
Figure 6 Expression of key receptors involved in the uptake of ceramic NCs.
Notes: (A) Significant levels of increased iron in treatment groups (red bars) compared to control (blue) in case of spleen, liver, intestine, brain and tumor were observed. (B) Lower left panel shows control group negative for Prussian blue staining. (C) Inset shows internalization of Fe-bLf–loaded ACSC NCs near the CD31-stained area. (D) miRNA expression analysis from intestine, liver, spleen, and brain was carried out in Fe-bLF-NC–treated mice. (E) LRPI expression was comparatively higher in vitro when compared to in vivo. LRP2 mRNA levels were elevated in mice liver, brain, and intestine tissues. (F) Expression of TfR and its isoforms was high in MDA cells, liver, brain, and intestinal tissues. Data represent mean ± standard error of the mean. Experiments were repeated three times independently with similar results. **P<0.005; *P<0.05.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; LRP, low-density lipoprotein receptor; miRNA, micro-RNA; NCs, nanocapsules/nanocarriers; TfR, transferrin receptor.
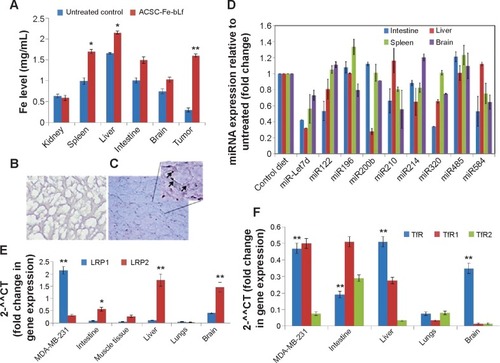
Table 2 Serum iron, serum calcium, and blood hematological profile of control and experimental mice with bovine lactoferrin–fed groups and mice fed on control diet
Figure 7 Mechanism of uptake of ACSC-Fe-bLf.
Notes: The double coating of alginate and chitosan protects (ACSC-Fe-bLf) Fe-bLF from acidic degradation in the stomach. The CSC-Fe-bLf NCs also lead to an upregulation of miRNAs responsible for iron metabolism mainly miR196, miR200b, and miR485 in the intestine. This enhances their uptake by the intestinal cells. Because Fe-bLf is a ligand for TfR and low-density lipoprotein receptor, the CSC-Fe-bLf NCs are endocytosed in the cancer cells, using these receptors. Control release of Fe-bLf from the NCs executes the antitumor efficacy by interfering with molecular mechanisms involved in the cellular apoptosis.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; Ca-P, calcium phosphate; EPR, enhanced permeability and retention; Fe-bLf, iron-saturated bovine lactoferrin; LRP, low-density lipoprotein receptor; miRNA, micro-RNA; NCs, nanocapsules/nanocarriers; TfR, transferrin receptor.
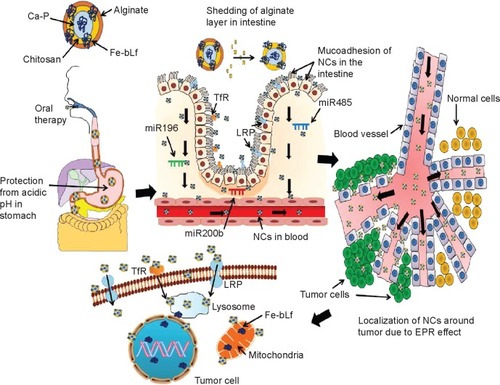
Figure S1 Time dependent internalization of NCs.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; CSC, chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; NCs, nanocapsules/nanocarriers.
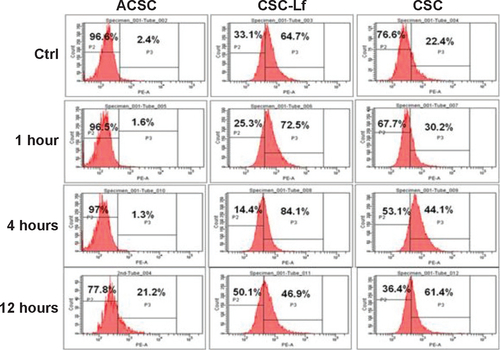
Figure S2 Determining the percentage viability in MDA-MB2-31 cells with NC treatments at 10 h.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; CSC, chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; h, hours; NC, nanocapsule/nanocarrier.
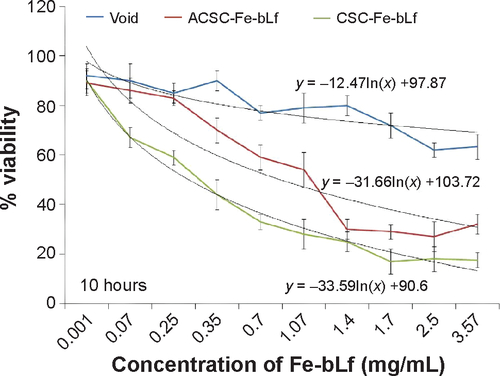
Figure S3 Determining the percentage viability in MDA-MB2-31 cells with NC treatments at 20 h.
Abbreviations: ACSC, alginate-enclosed chitosan-coated calcium phosphate; CSC, chitosan-coated calcium phosphate; h, hours; Lf, lactoferrin; NC, nanocapsule/nanocarrier.
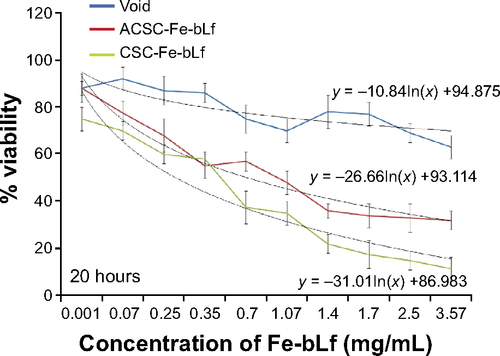
Figure S4 Determining the clonogenic potential of MDA-MB-231 with NC treatments.
Notes: (A) Clonogenic potential of MDA-MB-231 was performed. (B) It was revealed that the CSC-Fe-bLf NCs led to a significant reduction (*P≤0.05) in 12 and 24 h and a highly significant reduction (**P≤0.005) in 48, 72, and 96 h.
Abbreviations: CSC, chitosan-coated calcium phosphate; Fe-bLf, iron-saturated bovine lactoferrin; h, hours; NCs, nanocapsules/nanocarriers.
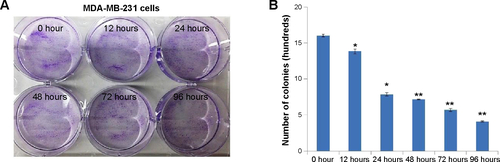
Figure S5 Agarose gel images of the Q-RT-PCR (quantitative real time polymerase chain reaction) products.
Abbreviation: LRP, low-density lipoprotein receptor.
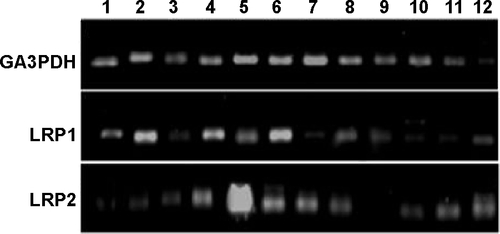
Figure S6 Agarose gel images of the Q-RT-PCR (quantitative real time polymerase chain reaction) of TfR and its isoforms.
Abbreviation: TfR, transferrin receptor.
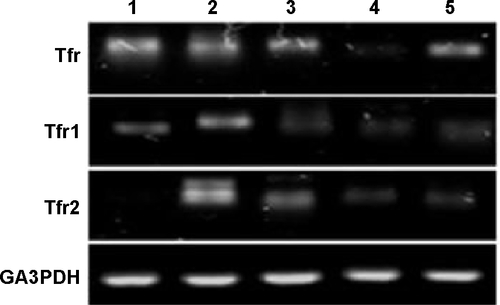
Table S1 Comparison of physicochemical and biological characters in CSC-Fe-bLf NCs and ACSC-Fe-bLf NCs
