Figures & data
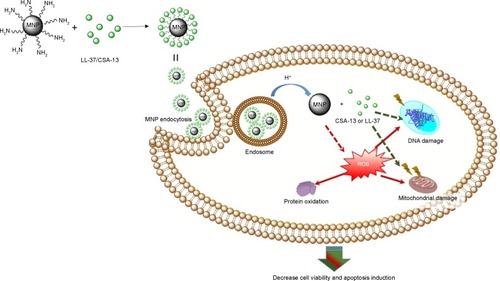
Figure 1 Attenuated total reflection Fourier transform infrared spectra of magnetic nanoparticles coated by human cathelicidin LL-37 (A) or the antimicrobial peptide analog ceragenin CSA-13 (B).
Abbreviations: MNP@LL-37, LL-37 peptide linked to magnetic nanoparticles; MNP@CSA-13, CSA-13 linked to magnetic nanoparticles.
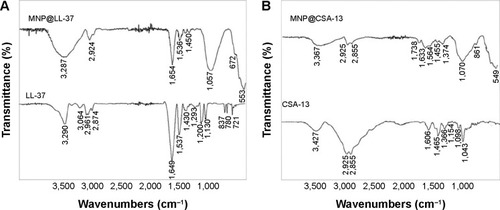
Figure 2 Ceragenin-coated magnetic nanoparticles decrease viability and induce apoptosis in DLD-1 cells. MTT assay of the viability of DLD-1 cells treated with derivatives of cathelicidin LL-37 (A) and ceragenin CSA-13 (B) at different concentrations for 24 hours. Apoptosis of DLD-1 cell treated with cathelicidin LL-37 (D) and ceragenin CSA-13 (E). Proliferation assay of DLD-1 cells treated with cathelicidin LL-37 (C) and ceragenin CSA-13 derivatives (F).
Note: *Significantly different compared with control (n=3–6).
Abbreviations: MNP, Magnetic Nanoparticles; MNP@LL-37, LL-37 peptide linked to magnetic nanoparticles; MNP@CSA-13, CSA-13 linked to magnetic nanoparticles; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
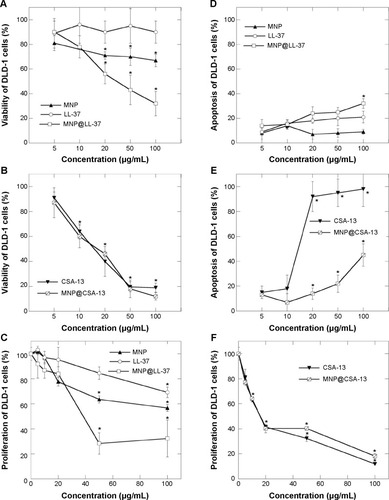
Figure 3 Cathelicidin LL-37-coated and ceragenin CSA-13-coated magnetic nanoparticles decrease viability, induce apoptosis, and decrease proliferation of HT-29 cells. Viability assay of HT-29 cells treated with derivatives of cathelicidin LL-37 (A) and ceragenin CSA-13 (B) at different concentrations for 24 hours. Apoptosis assay of HT-29 cells after treatment with cathelicidin LL-37 (D) or ceragenin CSA-13 (E) derivatives. Incorporation of 3H-thymidine into HT-29 cells treated with derivatives of cathelicidin LL-37 (C) or ceragenin CSA-13 (F) for 24 hours at different concentrations.
Note: *Significantly different compared with control (n=3–6).
Abbreviations: MNP, Magnetic Nanoparticles; MNP@LL-37, LL-37 peptide linked to magnetic nanoparticles; MNP@CSA-13, CSA-13 linked to magnetic nanoparticles; CT, control.
Figure 4 Ceragenin-coated and cathelicidin-coated magnetic nanoparticles induce apoptotic and necrotic changes in HT-29 cells. Microscopy evaluation revealed the normal structure of untreated HT-29 cells without any apoptotic or necrotic changes after 24 hours (A). Apoptotic features, including cell blebbing (BL) were observed after 24 hours of incubation with MNPs (D) or ceragenin CSA-13 (F). Clear markers of late apoptosis and necrosis were detected by appearance of an orange and red color after treatment with MNP@CSA-13 (B), MNP@LL-37 (C), MNP (D), and CSA-13 (F). Uncharacteristic changes were observed after treatment with LL-37 (E).
Notes: The image of the stained HT-29 cell population was acquired using the BD Pathway confocal microscope. Green indicates living cells and orange/red indicates apoptotic or necrotic cells.
Abbreviations: BL, membrane blebbing; LA, late apoptosis; N, necrosis; MNP, magnetic nanoparticles.
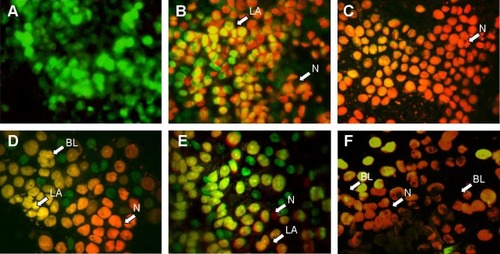
Figure 5 Internalization of the dual-labeled magnetic nanosystem (PI-MNP@LL-37-FITC or PI-MNP@CSA-13-FITC) into colorectal cancer HT-29 cells. This experiment was conducted using aminosilane-coated MNPs in which cathelicidin LL-37 or ceragenin CSA-13 attached to the MNP surface were linked to FITC. Additionally, aldehyde groups coating the MNP surface were labeled with PI. Schematic representation of dual-labeled nanosystems (A); FITC is indicated as green stars; PI is indicated as red stars. Internalization of cathelicidin LL-37-FITC (B) or PI-MNP@LL-37-FITC (C), ceragenin CSA-13-FITC (D) and PI-MNP@CSA-13-FITC (E). As indicated by arrows, both nanosystems were localized in the cytoplasm and/or nuclear compartment. (C, E) In some cells, LL-37-FITC and CSA-13-FITC molecules were released from the MNP surface (green fluorescence) and were localized in the nucleus area while the core–shell nanostructures were present in the cytosol (red fluorescence). Magnification 400×.
Abbreviations: CSA-13-FITC, ceragenin CSA-13 labeled by FITC; LL-37-FITC, cathelicidin LL-37 labeled by FITC; FITC, fluorescein isothiocyanate; PI, propidium iodide; MNP, magnetic nanoparticles; PI-MNP@LL-37-FITC/PI-MNP@CSA-13-FITC, dual-labeled magnetic nanosystems.
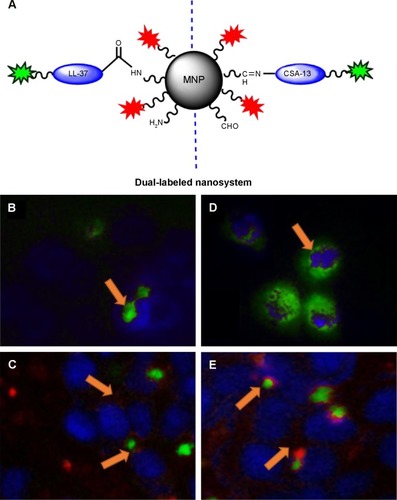
Figure 6 Hypothetical mechanism of anticancer activity of MNP@LL-37 or MNP@CSA-13. LL-37 was linked to the aminosilane MNPs by covalent bonds to construct nanosystems acting as a drug delivery system. MNPs, as drug carriers, increased LL-37 or CSA-13 chemotherapeutic efficiency after endocytosis and acid hydrolysis in the cell cytosol.
Abbreviations: MNP, magnetic nanoparticles; ROS, reactive oxygen species.