Figures & data
Figure 1 Methodology of the scaffold fabrication. Poly-ε-caprolactone (PCL) nanofibers were prepared by an electrospinning method. Electrospun nanofibers were deposited on a polypropylene (PP) surgical mesh, which was attached to the grounded collecting electrode from each side. PP covered with PCL nanofibers was cut into round patches of 6 mm in diameter, sterilized, and immersed in thrombocytes-rich solution (TRS) for 2 hours. The nonadhered thrombocytes were removed by rinsing twice in phosphate-buffered saline. The composite scaffolds were placed in a new well, seeded with 3T3 fibroblasts and tested in vitro.
Notes: (1) Syringe and metering pump, (2) needle serving as the electrode, (3) stable part of the jet, (4) whipping/coiling zone, (5) collector covered with PP, (6) ground, and (7) high-voltage supply.
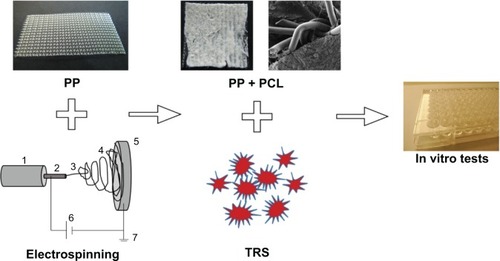
Figure 2 Scanning electron microscopy of the scaffolds. (A) Poly-ε-caprolactone (PCL) nanofibers, (B) polypropylene (PP) mesh, and (C) PP mesh functionalized with PCL nanofibers.
Notes: (A) Magnification ×230, scale bar 50 μm; (B and C) magnification ×18, scale bar 500 μm.

Figure 3 Cell adhesion evaluated on the 1st day after seeding. (1) Average surface area of spread 3T3 fibroblasts cultivated on the surface of a polypropylene (PP) mesh, (2) a PP mesh treated with thrombocyte-rich solution (TRS), (3) a PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, and (4) a PP mesh functionalized with PCL nanofibers treated with TRS. Cell adhesion assay revealed a significantly larger surface area of spread 3T3 fibroblasts on scaffolds functionalized with PCL nanofibers (PP + PCL and PP + PCL + TRS) than on scaffolds without functionalization (PP and PP + TRS). Moreover, the average surface area of 3T3 fibroblasts was significantly higher (level of significance at a value of P<0.001) on the PP mesh functionalized with PCL nanofibers treated with TRS than on all other scaffolds.
Notes: The level of statistical significance for the assays is designated above the mean values (P<0.05 indicated by number; P<0.001 indicated by number and *). Day 1: 2>1*; 3>1*, 2; 4>1*, 2*, 3*.
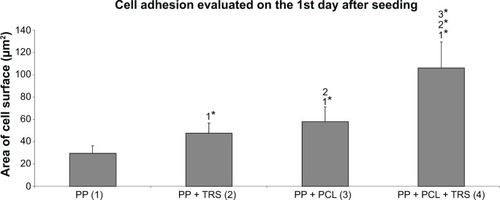
Figure 4 Metabolic activity of 3T3 fibroblasts cultivated on the surface of (1) polypropylene (PP) mesh, (2) PP mesh enriched with adhered thrombocytes, (3) PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, and (4) PP mesh functionalized with PCL nanofibers enriched with adhered thrombocytes. 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay revealed significantly higher metabolic activity of 3T3 fibroblasts on scaffolds functionalized with PCL nanofibers (PP + PCL and PP + PCL + thrombocyte-rich solution [TRS]) on Day 14 than on scaffolds without functionalization (PP). Moreover, the metabolic activity of 3T3 fibroblasts on Days 7, 10, and 14 was significantly higher on the PP mesh functionalized with PCL nanofibers treated with TRS than on all other scaffolds.
Notes: The level of statistical significance for the assays is designated above the mean values (P<0.05 indicated by a number; P<0.001 indicated by a number and *). Day 1: without significance. Day 3: 3>1, 2; 4>1*, 2*. Day 7: 3>1; 4>1*, 2*, 3. Day 10: 2>1; 3>1*; 4>1*, 2, 3. Day 14: 2>1*; 3>1*, 2*; 4>1*, 2*, 3.
![Figure 4 Metabolic activity of 3T3 fibroblasts cultivated on the surface of (1) polypropylene (PP) mesh, (2) PP mesh enriched with adhered thrombocytes, (3) PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, and (4) PP mesh functionalized with PCL nanofibers enriched with adhered thrombocytes. 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay revealed significantly higher metabolic activity of 3T3 fibroblasts on scaffolds functionalized with PCL nanofibers (PP + PCL and PP + PCL + thrombocyte-rich solution [TRS]) on Day 14 than on scaffolds without functionalization (PP). Moreover, the metabolic activity of 3T3 fibroblasts on Days 7, 10, and 14 was significantly higher on the PP mesh functionalized with PCL nanofibers treated with TRS than on all other scaffolds.Notes: The level of statistical significance for the assays is designated above the mean values (P<0.05 indicated by a number; P<0.001 indicated by a number and *). Day 1: without significance. Day 3: 3>1, 2; 4>1*, 2*. Day 7: 3>1; 4>1*, 2*, 3. Day 10: 2>1; 3>1*; 4>1*, 2, 3. Day 14: 2>1*; 3>1*, 2*; 4>1*, 2*, 3.](/cms/asset/8fadf608-53fa-4c00-98db-eb4a234f7310/dijn_a_77816_f0004_b.jpg)
Figure 5 Proliferative activity of 3T3 fibroblasts cultivated on the surface of (1) polypropylene (PP) mesh, (2) PP mesh enriched with adhered thrombocytes, (3) PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, and (4) PP mesh functionalized with PCL nanofibers treated with thrombocyte-rich solution (TRS). The PicoGreen® assay revealed significantly higher proliferation of 3T3 fibroblasts on scaffolds functionalized with PCL nanofibers (PP + PCL and PP + PCL + TRS) on Days 7, 10, and 14 than on scaffolds without functionalization (PP and PP + TRS). In addition, the proliferation of 3T3 fibroblasts on Days 7, 10, and 14 was significantly higher (level of significance at value of P<0.001) on the PP mesh functionalized with PCL nanofibers enriched with adhered thrombocytes than on all other scaffolds.
Notes: The level of statistical significance for the assays is designated above the mean values (P<0.05 indicated by a number; P<0.001 indicated by a number and *). Day 1: 4>1, 2, 3. Day 3: 3>1, 2; 4>1, 2. Day 7: 3>1, 2; 4>1*, 2*, 3*. Day 10: 2>1*; 3>1*, 2*; 4>1*, 2*, 3*. Day 14: 2>1; 3>1*, 2; 4>1*, 2*, 3*.
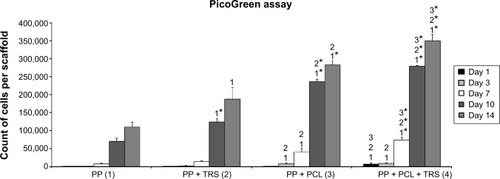
Figure 6 Viability of 3T3 fibroblasts cultivated on the surface of (A) polypropylene (PP) mesh, (B) PP mesh treated with thrombocyte-rich solution (TRS), (C) PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, (D) PP mesh functionalized with PCL nanofibers treated with TRS, (E) PCL nanofibers, and (F) PCL nanofibers treated with TRS on Day 14 after seeding. Live/dead cell staining revealed a higher percentage of viable cells on all scaffolds functionalized either with PCL nanofibers or with TRS than on the scaffold without any functionalization or treatment (PP). The percentages of viable cells cultivated on the various surfaces of the scaffolds were: (A) 59.5%, (B) 85.4%, (C) 88.3%, (D) 90.1%, (E) 90.3%, and (F) 94.7%.
Notes: The viability of 3T3 fibroblasts was evaluated on Days 1, 3, 7, 10, and 14 after seeding. For simplification, only data obtained on Day 14 are presented. Viability was calculated as the percentage of live cells from the total cell count per unit area. Live cells are stained green. Dead cells are stained red. Scale bar 200 μm.
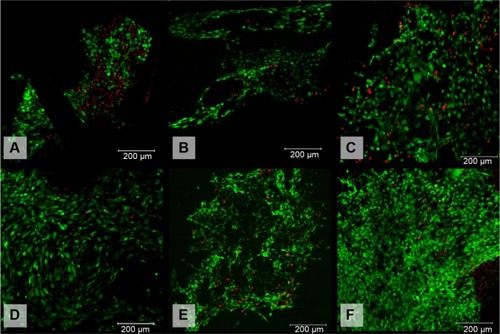
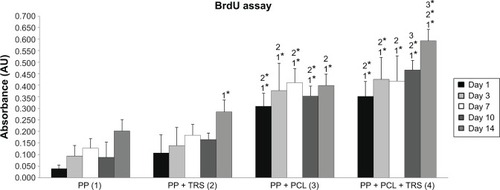
Figure 7 Proliferation of 3T3 fibroblasts cultivated on the surface of (1) polypropylene (PP) mesh, (2) PP mesh treated with thrombocyte-rich solution (TRS), (3) PP mesh functionalized with poly-ε-caprolactone (PCL) nanofibers, and (4) PP mesh functionalized with PCL nanofibers treated with TRS. A 5-bromo-2′-deoxyuridine (BrdU) colorimetric immunoassay revealed significantly greater proliferation of 3T3 fibroblasts on scaffolds functionalized with PCL nanofibers (PP + PCL and PP + PCL + TRS) on all days of evaluation than on scaffolds without functionalization (PP and PP + TRS). In addition, the proliferation of 3T3 fibroblasts on Day 14 was significantly higher (P<0.001) on the PP mesh functionalized with PCL nanofibers treated with TRS than on all other scaffolds.
Notes: The level of statistical significance for the assays is designated above the mean values (P<0.05 indicated by a number, P<0.001 indicated by a number and *). Day 1: 3>1*, 2*; 4>1*, 2*. Day 3: 3>1*, 2; 4>1*, 2*. Day 7: 3>1*, 2*; 4>1*, 2. Day 10: 3>1*, 2*; 4>1*, 2*, 3. Day 14: 2>1*; 3>1*, 2; 4>1*, 2*, 3*.
Abbreviation: AU, absorbance units.