Figures & data
Table 1 Selected FDA-approved nanodrugs
Table 2 Examples of NP-based carriers for brain-targeted drug delivery
Figure 1 Self-templating process for the formation of silica microcapsules with a thin flake–shell architecture.
Notes: Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Reprinted from Ji QM, Guo CY, Yu XY, et al. Flake–shell capsules: adjustable inorganic structures. Small. 2012;8(15):2345–2349.Citation148 The flake–shell is formed by dissolving the silica nanoparticles on the outside and precipitation/aggregation of nanosheets in the surrounding area.
Figure 2 Mechanisms of NP-induced ROS generation in cells.
Notes: NPs can provoke oxidative stress through multiple interactions: 1) the interaction of NPs with mitochondria leads to mitochondria dysfunction; 2) direct interaction occurs with cytoplasmic enzymes responsible for maintaining cellular redox potential, such as NADPH; 3) the activation of intracellular signaling cascades induces the formation of ROS; 4) degradation of the NP coating and core in the lysosomal environment leads to lysosome dysfunction, such as LMP; and 5) some metal-based NPs can generate ROS via Fenton-like reactions.
Abbreviations: NP, nanoparticle; ROS, reactive oxygen species; LMP, lysosomal membrane permeabilization.
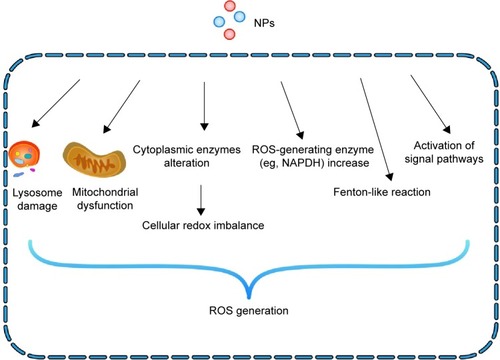
Figure 3 Overview of the mechanistic steps of NP-induced autophagy.
Notes: NPs induce autophagy in two ways: ROS-dependent autophagy and lysosome-dependent autophagy. During autophagy, a phagophore is created and it then elongates into a double-membrane autophagosome while sequestering cytoplasmic material. This autophagosome then fuses with a lysosome, resulting in an autolysosome. The enzymes present in the autolysosome lumen eventually degrade the inner membrane and autophagic cargo, thus providing macromolecules that can be transported into the cytosol via permeases. TFEB is a transcription factor that translocates to the nucleus upon activation (similar to dysfunction of the lysosome), where it promotes the transcription of the lysosomal and autophagic genes. Proper stimulation will help cells survive; however, under- and overstimulation of autophagy will lead to cell death.
Abbreviations: NP, nanoparticle; TFEB, transcription factor EB; ROS, reactive oxygen species; Atg, autophagy protein.
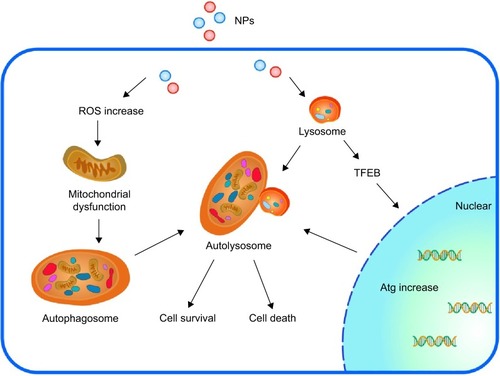
Figure 4 ZnO NPs trigger autophagy.
Notes: (A) TEM images of autophagosomes and cellular structures in macrophages treated with ZnO NPs. Black arrows point to ZNP clusters. Autophagosome formations in ZnO NP-treated cells are indicated by red arrows. High-magnification view of a large autolysosome containing clusters of ZnO NPs and cellular debris. Nuclei of the treated cells also contain large numbers of dense ZnO NPs. (B) Detection of autophagic vacuoles in macrophages at different time points (control, 0.5, 3, 6, 12, and 24 hours [h]), treated cells and rapamycin (positive control)-treated cells. (C) Autophagic flux in macrophages under ZnO NP exposure measured by staining with LC3-FITC antibody and assessed by a fluorimeter. Reprinted from Toxicol Lett; 227(1), Roy R, Singh SK, Chauhan KS, Das M, Tripathi A, Dwivedi PD, Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition, 29–40, Copyright © 2014 with permission from Elsevier Ireland Ltd.Citation103
Abbreviations: ZnO NPs, zinc oxide nanoparticles; TEM, transmission electron microscopy; FITC, Fluorescein isothiocyanate isomer I.
![Figure 4 ZnO NPs trigger autophagy.Notes: (A) TEM images of autophagosomes and cellular structures in macrophages treated with ZnO NPs. Black arrows point to ZNP clusters. Autophagosome formations in ZnO NP-treated cells are indicated by red arrows. High-magnification view of a large autolysosome containing clusters of ZnO NPs and cellular debris. Nuclei of the treated cells also contain large numbers of dense ZnO NPs. (B) Detection of autophagic vacuoles in macrophages at different time points (control, 0.5, 3, 6, 12, and 24 hours [h]), treated cells and rapamycin (positive control)-treated cells. (C) Autophagic flux in macrophages under ZnO NP exposure measured by staining with LC3-FITC antibody and assessed by a fluorimeter. Reprinted from Toxicol Lett; 227(1), Roy R, Singh SK, Chauhan KS, Das M, Tripathi A, Dwivedi PD, Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition, 29–40, Copyright © 2014 with permission from Elsevier Ireland Ltd.Citation103Abbreviations: ZnO NPs, zinc oxide nanoparticles; TEM, transmission electron microscopy; FITC, Fluorescein isothiocyanate isomer I.](/cms/asset/bd3a7f33-b93d-4e51-baca-3993650c1d56/dijn_a_78308_f0004_c.jpg)
