Figures & data
Figure 2 Schematic description of the synthesis of TPGS-b-PCL diblock copolymer.
Abbreviations: ε-CL, ε-caprolactone; h, hours; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
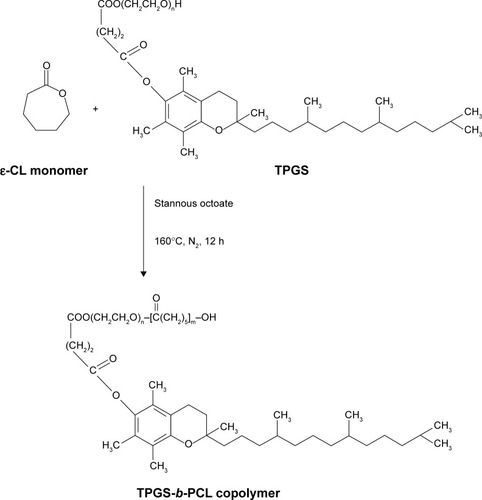
Figure 3 FTIR spectra of TPGS and TPGS-b-PCL copolymer.
Abbreviations: FTIR, Fourier transform infrared; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
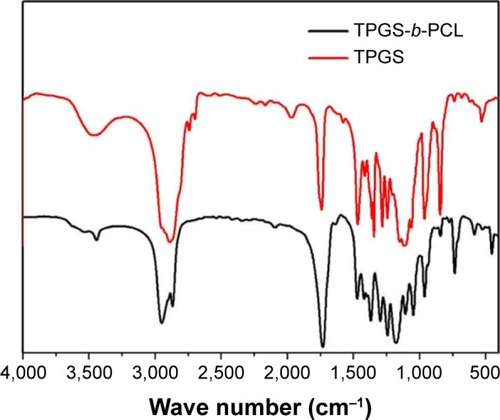
Figure 4 Typical 1H-NMR spectra of TPGS-b-PCL copolymer (A) and TPGS (B).
Abbreviations: 1H-NMR, proton nuclear magnetic resonance; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
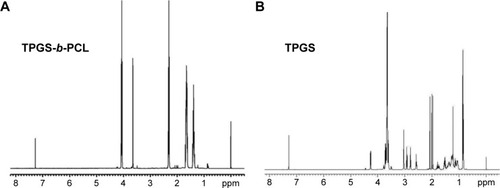
Figure 5 Characterization of TPGS-b-PCL copolymer.
Notes: (A) Typical gel permeation chromatograms of TPGS and TPGS-b-PCL diblock copolymer; (B) thermogravimetric profiles of TPGS and TPGS-b-PCL copolymer.
Abbreviations: min, minutes; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
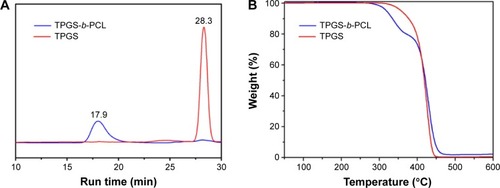
Table 1 Characterization of nanoparticles
Figure 6 FESEM image (A), TEM image (B), and DLS spectra (C) of genistein-loaded TPGS-b-PCL NPs.
Abbreviations: DLS, dynamic light scattering; FESEM, field emission scanning electron microscopic; NPs, nanoparticles; PCL, poly(ε-caprolactone); TEM, transmission electron microscopic; TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
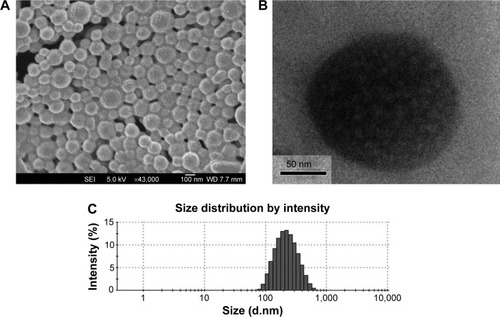
Figure 7 DSC thermograms of the pure genistein, blank PCL NPs, blank TPGS-b-PCL NPs, and genistein-loaded PCL NPs, and TPGS-b-PCL NPs.
Abbreviations: DSC, differential scanning calorimetry; ENDO, endotherm; NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
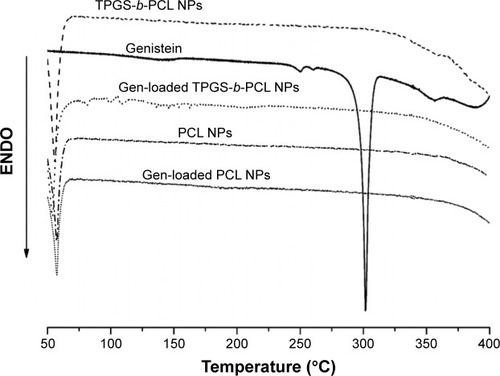
Figure 8 In vitro release profiles of genistein-loaded PCL NPs and TPGS-b-PCL NPs.
Abbreviations: NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
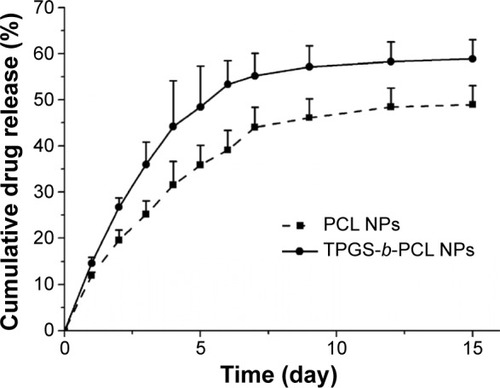
Figure 9 Cellular uptake of coumarin-6-loaded PCL NPs and TPGS-b-PCL NPs after incubated with HeLa cells.
Notes: (A) Uptake efficiency of coumarin-6-loaded PCL NPs and TPGS-b-PCL NPs by HeLa cells (*P<0.05); (B) confocal laser scanning microscopic images of HeLa cells after incubation with the coumarin-6-loaded TPGS-b-PCL NPs. Scale bar 10 μm.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
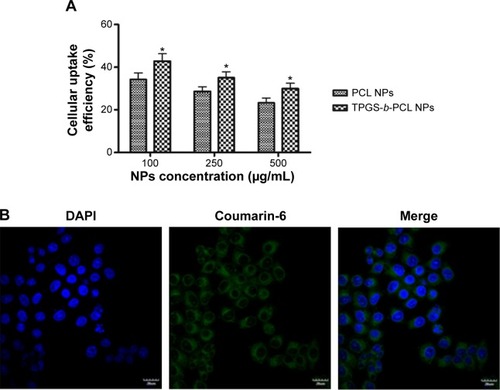
Figure 10 The anticancer activity of pristine genistein and genistein-loaded NPs on HeLa cervical cancer cells in vitro.
Notes: (A–C) The inhibitory effect of pristine genistein, genistein-loaded NPs, and drug-free NPs on the proliferation of HeLa cells at 24 h (A), 48 h (B), and 72 h (C), determined by MTT assay (*P<0.05); (D) the inhibitory effect of pristine genistein, genistein-loaded NPs, and drug-free NPs on the colony formation of HeLa cells; (E) colonies were counted and the data are expressed as a percentage of control cells (*P<0.05).
Abbreviations: NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate; h, hours; MTT, 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
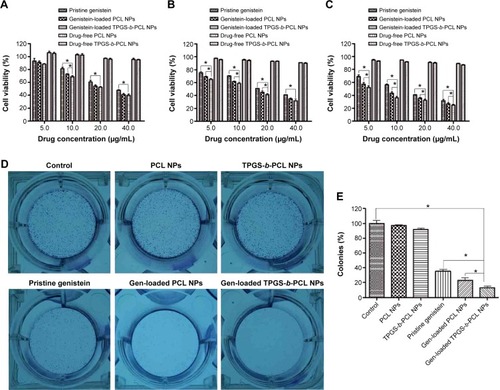
Table 2 IC50 values of pristine genistein, genistein-loaded PCL NPs, and genistein-loaded TPGS-b-PCL NPs on human cervical carcinoma cell line HeLa after 24, 48, and 72 hours of incubation
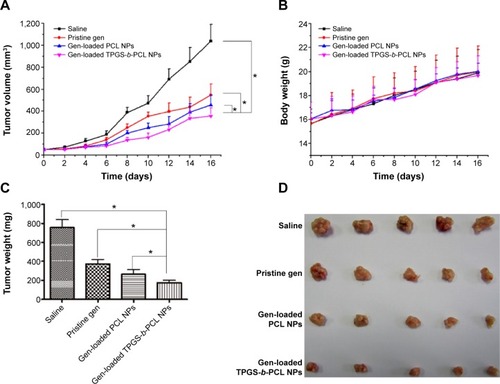
Figure 11 Antitumor effect of genistein formulated in TPGS-b-PCL in comparison with pristine genistein and genistein-loaded PCL NPs (n=5).
Notes: (A) Tumor growth curve of the BALB/c nude mice bearing HeLa cells xenograft after administration (*P<0.05); (B) body weight after administration; (C) weight of the tumor from each group taken out from the sacrificed mice at the end of the study (*P<0.05); (D) image of tumor from each group taken out from the sacrificed mice at the end of the study.
Abbreviations: NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.