Figures & data
Figure 1 Images of nanoparticles in suspension obtained by scanning electron microscopy at ×150,000.
Notes: (A) Nanogold; (B) nanosilver.
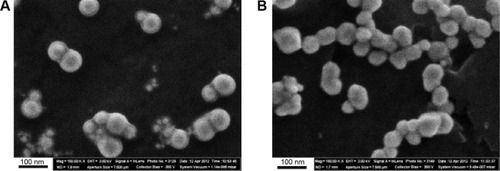
Figure 2 Nanoparticle (NP) size distribution function.
Notes: The results of statistical processing of 800 images of gold NPs (A) and 650 images of silver NPs (B) obtained by scanning electron microscopy.
Abbreviations: d, diameters of nanoparticles; N/N, number of nanoparticles.
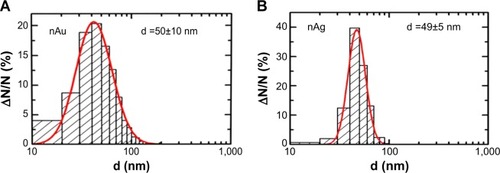
Table 1 Number of cells in the bronchoalveolar lavage fluid (BALF) 24 hours after the intratracheal instillation of silver nano- or microparticles to rats at a dose of 0.2 mg per rat (x±se)
Figure 3 The ratio of the number of neutrophil leukocytes (NL) to the number of alveolar macrophages (AM) in the bronchoalveolar lavage fluid of rats 24 hours after the instillation of magnetite particles of different sizes at a dose of 2 mg in 1 mL of distilled water (x±SX).
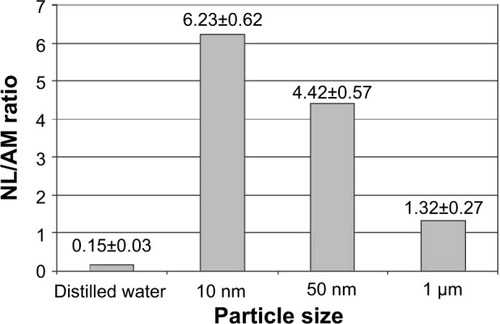
Figure 4 Percentage of phagocytic cells with different levels of particle burden in the bronchoalveolar lavage fluid of rats 24 hours after the intratracheal instillation of Fe3O4 particles having different diameters.
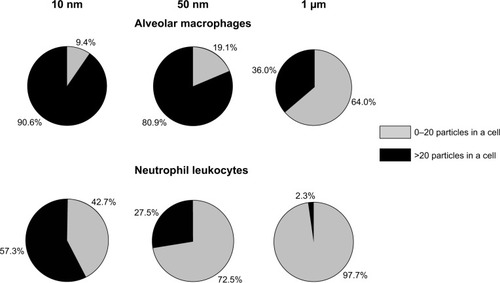
Figure 5 Alveolar macrophage surface topography measured by semi-contact atomic force microscopy.
Notes: (A) Control; (B) after instillation of 10 nm magnetite; (C) after instillation of 50 nm magnetite; (D) after instillation of 1 μm magnetite particles.
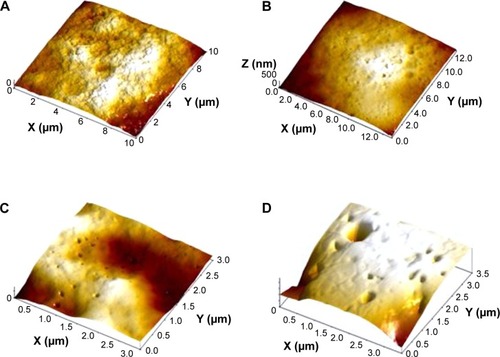
Figure 6 Alveolar macrophage surface topography measured by semi-contact atomic force microscopy.
Notes: (A) Controls; (B) after instillation of 340 nm copper oxide; (C) after instillation of 20 nm copper oxide particles.
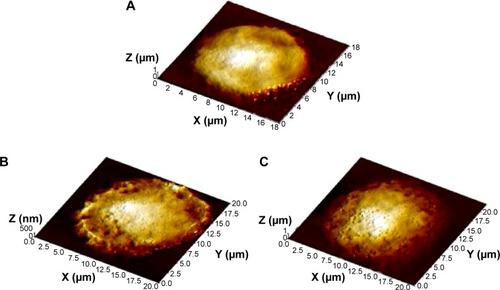
Figure 7 Average (x±SX) surface concentration of pits of all transverse dimensions detected on the surfaces of cells of each group of rats administered magnetite particles of different diameters and of control rats (Ref).
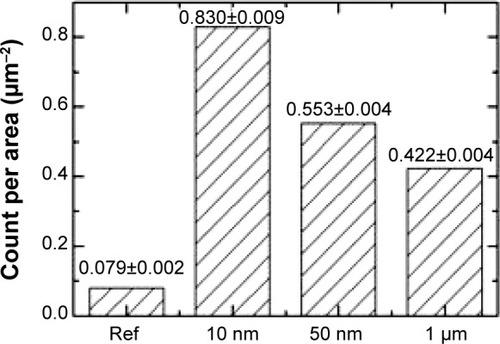
Figure 8 Engulfment of 10 nm magnetite particles by an alveolar macrophage.
Notes: Transmission electron microscopy, magnification ×140,000. Phagosomes are shown with arrows 1.
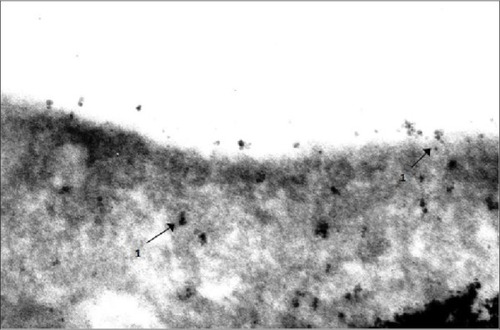
Table 2 Number of cells in the bronchoalveolar lavage fluid (BALF) 24 hours after the intratracheal instillation of suspension of gold (50 nm) or silver (49 nm) particles to rats at a dose of 0.2 mg per rat (x±se)
Figure 9 Gold nanoparticles uniformly distributed throughout the cytoplasm and nucleus of an alveolar macrophage.
Notes: The two-contour organization of the nucleus membrane is intact throughout. There is a mitochondrion visible which is not interacting with nanoparticles but, nevertheless, is intact only partly. Transmission electron microscopy, magnification ×22,000.
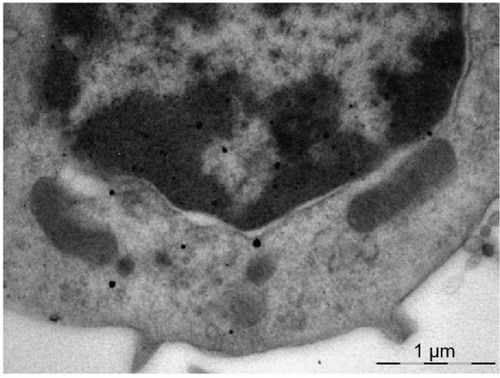
Figure 10 An alveolar macrophage.
Notes: Penetration of silver nanoparticles from aggregates in the cytoplasm into mitochondria (arrows). No silver nanoparticles are discovered in the nucleus. Transmission electron microscopy, magnification ×28,000.
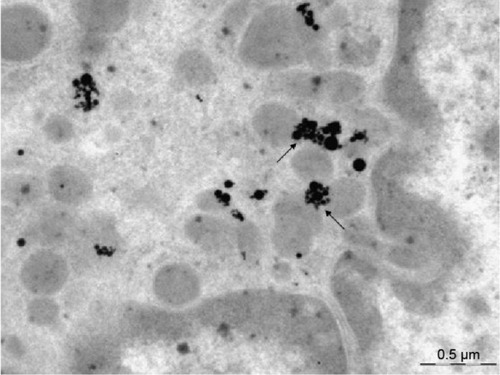
Table 3 Some morphometric indices for the state of rat’s liver after subchronic exposure to iron oxide (magnetite) particles (x±se)
Figure 11 Mean value (± standard deviation) of iron oxide (magnetite) concentration in the rat tissues of (A) liver and (B) spleen after repeated intraperitoneal injections of magnetite particles of different size.
Note: Electron paramagnetic resonance method.
Abbreviaton: NPs, nanoparticles.
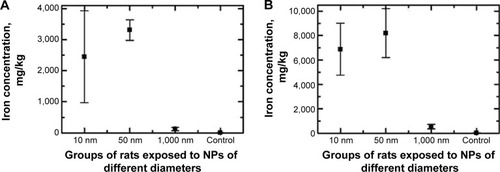
Table 4 Coefficients of the genomic DNA fragmentation in rats exposed to subchronic administration of silver or gold nanoparticles (based on the results of RAPD-test), x±se
Table 5 Succinate dehydrogenase activity (number of formasan granules per 50 blood lymphocytes) in rats exposed to subchronic administration of nanosilver particles with and without protection by a bio-protective complex (BPC) (x±se)
Table 6 Morphometric indices for tubular epithelium damage in kidneys of rats after repeat intraperitoneal injections of copper oxide nano-suspensions and/or oral administration of the bio-protective complex (BPC) (x±se)
Table 7 Coefficients of the genomic DNA fragmentation in rats exposed to subchronic administration of nanosilver particles with and without protection by a bio-protective complex (BPC) (based on the results of RAPD-test), x±se
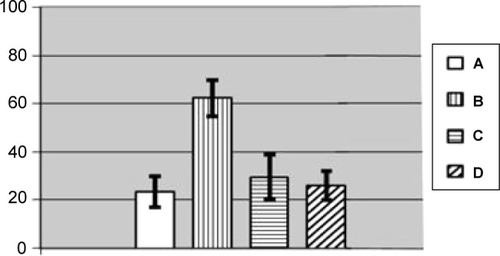
Figure 12 Number of brown pigment micro aggregates per square of Avtandilov’s grid in the spleen’s red pulp of rats exposed (A) to water (control); (B) to water suspension of copper oxide nanoparticles; (C) to the same against the background of BPC administrations; and (D) to the BPC only.
Notes: Average values with 95% confidence intervals. Differences are statistically significant between (B) or (C) versus (A), and between (C) versus (B) (P<0.05 by Student’s t-test).
Abbreviation: BPC, bio-protective complexes.
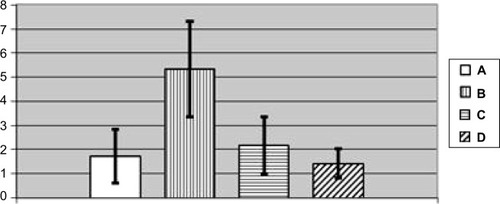
Figure 13 Number of cells without a nucleolus per 100 Golgi cells in nucleus caudatus of rats exposed (A) to water (control); (B) to water suspension of copper oxide nanoparticles; (C) to the same against the background of BPC administrations; (D) to the BPC only (average values with 95% confidence interval).
Notes: Differences are statistically significant between (B) and (A); (C) and (B) (P<0.05 by Student’s t-test).
Abbreviation: BPC, bio-protective complexes.