Figures & data
Figure 1 Schematic representation of the blood–brain barrier.
Notes: (A) Scheme of the neurovascular unit that constitutes the BBB. This unit contains the pericytes and astrocytes, which play an important role in the formation and differentiation of the CNS vasculature. (B) The main routes of the crossing of molecules to the CNS are represented. (a) A representation of the tight junctions severely restricting the penetration of water-soluble compounds, including polar drugs. (b) The endothelium contains transport proteins (carriers) for glucose, amino acids, purine bases, nucleosides, choline, and other substances. Some transporters are energy dependent (eg, P-glycoprotein) and act as efflux transporters (eg, azidothymidine). (c) An effective diffusive route for lipid-soluble agents to cross the membranes of the endothelium. (d) Certain proteins, such as insulin and transferrin, are taken up by specific receptor-mediated endocytosis and transcytosis. Nanoparticles capped with these molecules can cross the BBB. (e) Native plasma proteins such as albumin are poorly transported; however, cationization can increase their uptake by adsorptive-mediated endocytosis and transcytosis. (f) The efflux pump expulses the drugs from the endothelial cells to the blood. Delivery of nanoparticles across the brain endothelium enters mainly via routes (d) and (e). (g) Cytochrome P450 enzymes. Adapted from Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.Citation22
Abbreviations: BBB, blood–brain barrier; CNS, central nervous system.
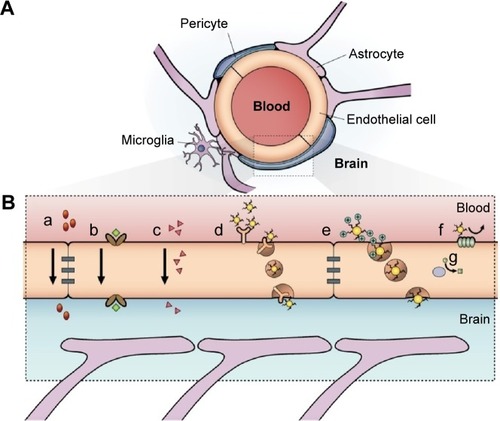
Figure 2 Schematic outline of the glymphatic system.
Notes: Convective glymphatic fluxes of cerebrospinal fluid and interstitial fluid propel the waste products of neuron metabolism into the para-venous space, from which they are directed into lymphatic vessels and ultimately return to the general circulation for clearance by the kidney and liver. From Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340(6140):1529–1530. Reprinted with permission from AAAS.Citation28
Abbreviations: CSF, cerebrospinal fluid; ISF, interstitial fluid.
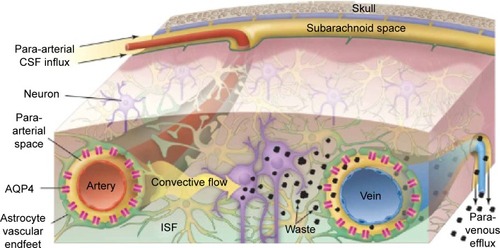
Table 1 Preclinical uses of peptides and proteins in the delivery of metal nanoparticles and their potential applications
Figure 3 Scheme to rationalize the design of the conjugate AuNP-THR-LPFFD.
Notes: (a) The peptide THR-CLPFFD anchored to the AuNP. This peptide contains the THR sequence, which recognizes the transferrin receptor, and LPFFD, which recognizes Aβ aggregates. (b) Transport of the endocytic vesicle through the endothelial cells of the BBB. (c) Recognition and binding of the conjugate to Aβ aggregates inside the CNS. The transferrin receptor is abbreviated as TfR. Reprinted from Prades R, Guerrero S, Araya E, et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33(29):7194–7205, with permission from Elsevier.Citation115
Abbreviations: AuNP, gold nanoparticle; BBB, blood–brain barrier; CNS, central nervous system.
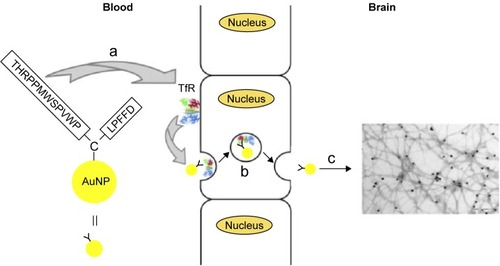
Figure 4 Amyloid plaques detected with in vivo mMRI after intravenous femoral injection of USPIO-PEG-Aβ1-42.
Notes: (A) In vivo T2*-weighted mMRI images of a 14-month-old APP/PS1 transgenic mouse. (B) A higher magnification of the area in the red box shown in (A). Arrowheads highlight some amyloid plaques detected by mMRI. (C) In vivo mMRI of a 16-month-old wild-type mouse after intravenous femoral injection of USPIO-PEG-Aβ1-42. (D) A higher magnification of the area in the red box shown in (C). Adapted with permission from Wadghiri YZ, Li J, Wang J, et al. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. PLoS One. 2013;8(2):e57097.Citation120
Abbreviations: mMRI, magnetic resonance microimaging; USPIO, ultrasmall superparamagnetic iron oxide; PEG, polyethylene glycol.
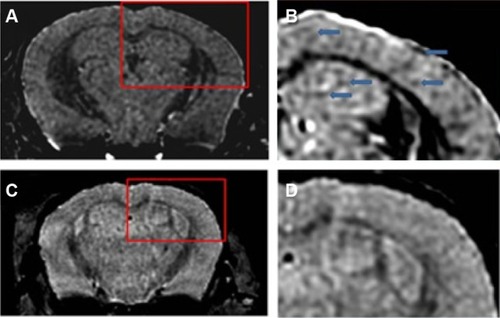
Figure 5 The increment of the uptake of AuNPs into the brain.
Notes: Left: The conjugate peptide-AuNPs labeled with 68Ga. Right: The increment of the uptake of AuNPs into the brain. ***P<0.001, significantly different from no targeted AuNP. Adapted with permission from Frigell J, Garcia I, Gomez-Vallejo V, Llop J, Penades S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J Am Chem Soc. 2014;136(1):449–457, Copyright 2014 American Chemical Society.Citation122
Abbreviations: AuNPs, gold nanoparticles; PET/CT, positron emission tomography/computed tomography.
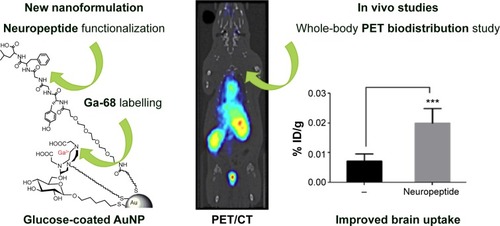
Figure 6 Two-photon luminescence imaging of internalized AuNPs in microglia and primary hippocampal neurons.
Notes: (A and B) Internalization of PEG- and CTAB-coated AuNPs in microglia. AuNPs are primarily found in the cytosol, and to a certain extent, colocalize with lysosomal compartments (indicated by enlarged inset image). Scale bar (20 µm) in control overlay image is representative for all panels. (B) Internalization of urchin AuNPs. Last column of panels provides a schematic illustrating the position along the z axis relative to the cell. Scale bar (5 µm) in the first panel is representative for all panels. (C) Two-photon luminescence imaging of primary hippocampal neurons treated after 21 days in vitro with (b) PEG-coated and (c) CTAB-coated GNPs for up to 4 hours. Neurons were stained with MitoTracker green FM (pseudocolored red). Untreated control cells can be seen in (a). Reprinted with permission from Hutter E, Boridy S, Labrecque S, et al. Microglial response to gold nanoparticles. ACS Nano. 2010;4(5):2595–2606, copyright 2010 American Chemical Society.Citation138
Abbreviations: AuNPs, gold nanoparticles; PEG, polyethylene glycol; CTAB, cetyl trimethylammonium bromide; GNPs, glucose-coated AuNPs.
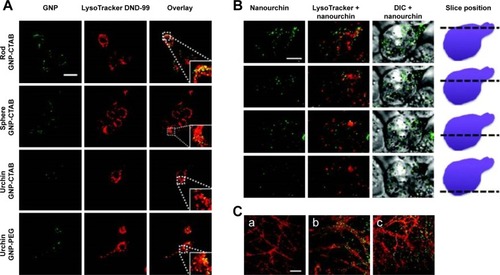
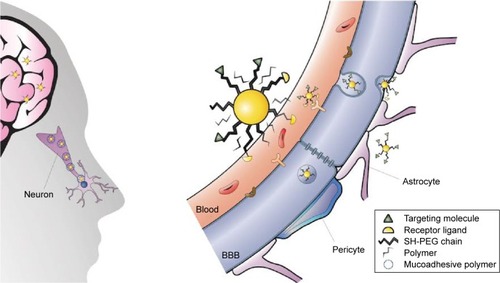
Figure 7 Strategies to enhance the delivery of AuNPs to the CNS.
Notes: Left: Intranasal administration of functionalized AuNPs that recognize the target in the brain. For the mucoadhesion of the nanoparticles to the mucosa, the nanoparticles could be capped with a polymer. Right: AuNPs capped with PEG diminish the interaction with plasma proteins, a peptide that recognizes the receptor (represented in yellow as a semicircle) for RMT and a peptide that recognizes the therapeutic target (green triangles). Note that AuNPs are not scaled with respect to the cells, brain, and organism.
Abbreviations: AuNPs, gold nanoparticles; CNS, central nervous system; PEG, polyethylene glycol; RMT, receptor-mediated transcytosis; BBB, blood–brain barrier.