Figures & data
Figure 1 Schematic of the bacteria study (Staphylococcus aureus, ATCC 29213).
Notes: Bacteria colonies were counted, and the mean was calculated before incubation with PCL–maghemite, PCL–hematite, PCL–CIP–maghemite, PCL–CIP–hematite, or control (bacteria in broth). Bacteria (1:105 cfu) in the broth were cultured with particles for up to 5 days. At days 1, 2, and 5, bacteria were plated in sheep blood agar plates and incubated overnight, and the colonies were counted.
Abbreviations: PCL, polycaprolactone; CIP, ciprofloxacin; PBS, phosphate-buffered saline.
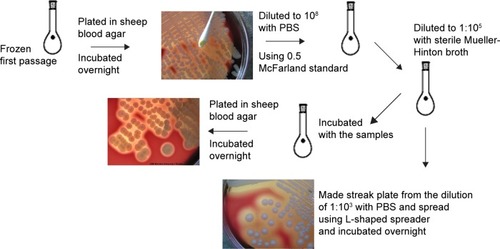
Figure 2 TEM micrographs of (A) maghemite (nonspray-dried iron oxide NPs), (B) hematite (spray-dried iron oxide NPs), (C) PCL–CIP–maghemite microsphere, and (D) PCL–CIP–hematite microsphere.
Abbreviations: TEM, transmission electron microscope; NPs, nanoparticles; PCL, polycaprolactone; CIP, ciprofloxacin.
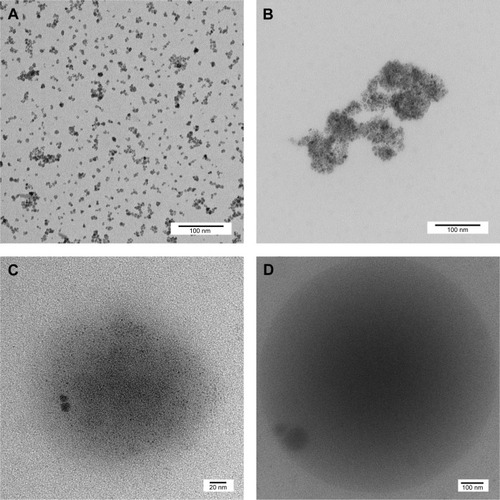
Figure 3 Raman spectra of maghemite (γ-Fe2O3, nonspray-dried iron oxide NPs) and hematite (α-Fe2O3, spray-dried iron oxide NPs).
Abbreviation: NPs, nanoparticles.
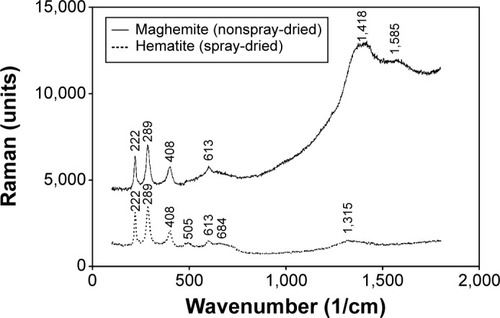
Figure 4 ATR-FTIR spectra of (A) maghemite (nonspray-dried iron oxide NPs) and hematite (spray-dried iron oxide NPs), (B) PCL and CIP, (C) PCL–CIP–maghemite microspheres, and (D) PCL–CIP–hematite microspheres.
Abbreviations: ATR–FTIR, attenuated total reflectance–Fourier transform infrared; NPs, nanoparticles; PCL, polycaprolactone; CIP, ciprofloxacin.
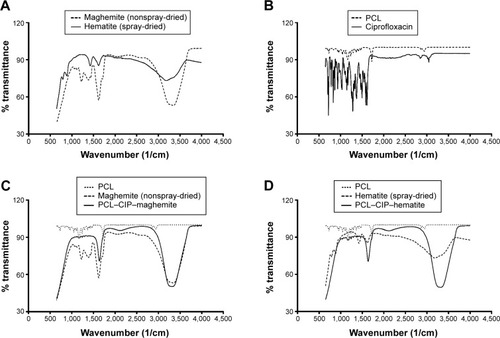
Figure 5 Histograms of the hydrodynamic size distribution of (A) maghemite (γ-Fe2O3, nonspray-dried NPs), (B) hematite (α-Fe2O3, spray-dried iron oxide NPs), (C) PCL–CIP–maghemite, and (D) PCL–CIP–hematite.
Note: Values are mean ± standard error of the mean; N=3.
Abbreviations: NPs, nanoparticles; PCL, polycaprolactone; CIP, ciprofloxacin.
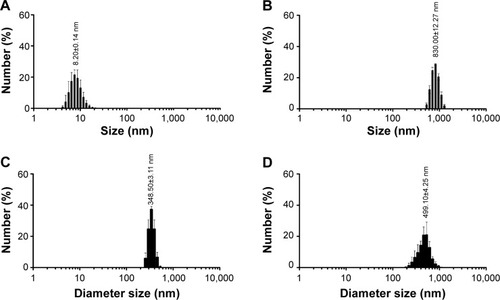
Figure 6 Staphylococcus aureus in broth inhibition assay in (A) mean count (cfu/mL) and (B) relative count with the treatments of PCL–CIP–maghemite and PCL–CIP–hematite microspheres, and those in combination with AMF.
Notes: Controls were bacteria in broth without any treatment. Data are represented at days 1, 2, and 5 of incubation. Values are mean ± standard error of the mean; n=3, and P-values were calculated using one-way ANOVA with post hoc Tukey’s test. +P<0.05 when compared with the data at day 5 of the samples, including PCL–CIP–hematite, PCL–CIP–maghemite, and PCL–CIP–maghemite with AMF.
Abbreviations: PCL, polycaprolactone; CIP, ciprofloxacin; AMF, alternating magnetic field; ANOVA, analysis of variance.
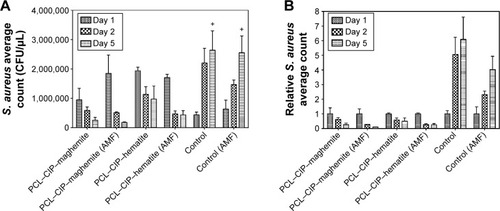
Figure 7 Fibroblast and macrophage viability was analyzed using an MTS assay in the presence of maghemite, hematite, PCL–CIP–maghemite, and PCL–CIP–hematite.
Notes: (A) Fibroblasts were cultured for 1 day to assess cell viability and (B) macrophages were incubated for 56 hours to assess the inflammation response. The percentages were calculated based on the control group (without any particles). Values are mean ± standard error of the mean; n=3. The P-values were calculated using a one-way ANOVA with post hoc Tukey’s test. *P<0.05 when compared with maghemite, hematite, and PCL–CIP–maghemite at the same concentration.
Abbreviations: PCL, polycaprolactone; CIP, ciprofloxacin; ANOVA, analysis of variance.
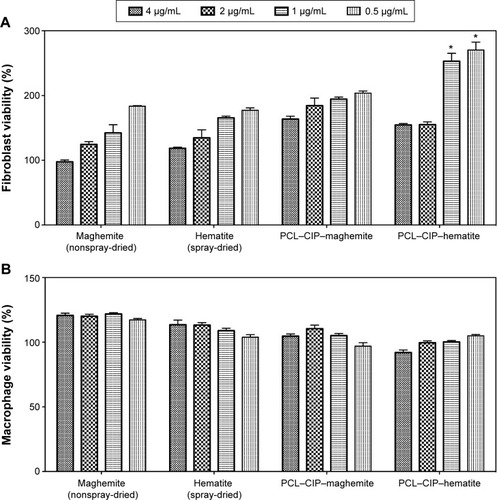
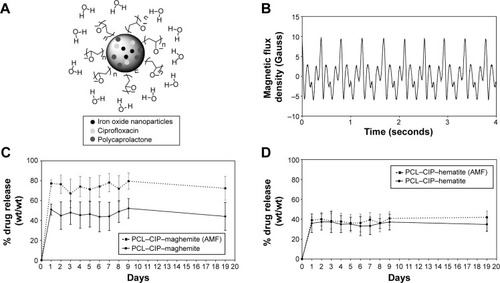
Figure 8 (A) Schematics of PCL, maghemite or hematite, and CIP in the oil sphere and water phases. (B) AMF was generated using a bed magnet placed on a laboratory rotator (orbital diameter =0.75 in) at 220 rpm. The suspension of bacteria and PCL–CIP–maghemite or PCL–CIP–hematite microspheres was incubated in the presence of AMF to study the release of CIP from the PCL–CIP–maghemite and PCL–CIP–hematite microspheres. (C, D) Representative curves of the percentage of CIP released from PCL–CIP–maghemite and PCL–CIP–hematite microspheres with and without AMF in PBS (1 M, pH 7.4). The microspheres were incubated at 37°C for up to 19 days. Values are mean ± standard error of mean; n=3.
Abbreviations: PCL, polycaprolactone; CIP, ciprofloxacin; AMF, alternating magnetic field; PBS, phosphate-buffered saline.