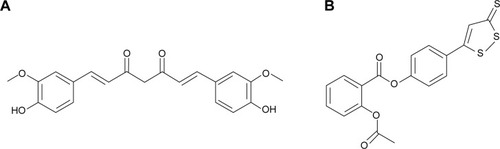
Figures & data
Table 1 Characterization of the prepared SH-ASA/Cur nanoparticles
Figure 2 Structure of an SH-ASA/curcumin-coloaded mPEG-PLGA nanoparticle.
Abbreviations: mPEG-PLGA, methoxy poly(ethylene glycol)-poly (lactide-coglycolide); SH-ASA, SH-aspirin.
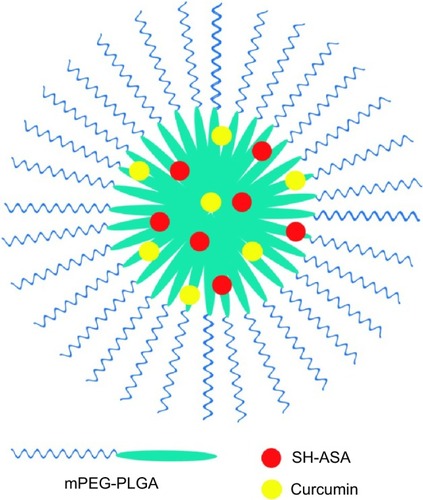
Figure 3 Characterization of SH-ASA/Cur-coloaded mPEG-PLGA nanoparticles.
Notes: (A) Size distribution; (B) zeta potential; (C) TEM image.
Abbreviations: SH-ASA, SH-aspirin; Cur, curcumin; mPEG-PLGA, methoxy poly(ethylene glycol)-poly (lactide-coglycolide); TEM, transmission electron microscope.
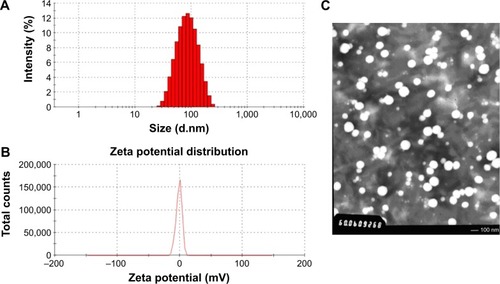
Figure 4 Characterization of SH-ASA/Cur-coloaded mPEG-PLGA NPs.
Notes: (A) DSC curves of blank NPs, Cur NPs, SH-ASA NPs, SH-ASA/Cur-coloaded NPs, SH-ASA NPs physically mixed with free Cur, Cur NPs mixed with free SH-ASA, and blank NPs mixed with both free drugs. (B) In vitro drug release behaviors of SH-ASA/Cur-coloaded mPEG-PLGA NPs.
Abbreviations: NP, nanoparticle; Cur, curcumin; SH-ASA, SH-aspirin; h, hours; mPEG-PLGA, methoxy poly(ethylene glycol)-poly (lactide-coglycolide); DSC, differential scanning calorimeter.
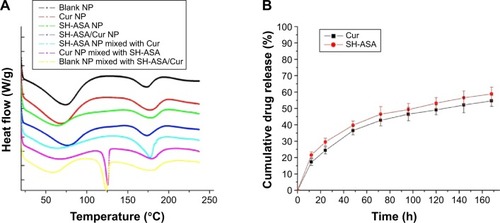
Figure 5 Uptake by SKOV3 cells.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Cur, curcumin; SH-ASA, SH-aspirin.
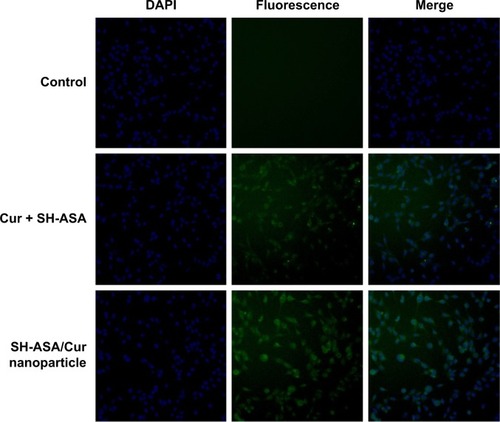
Figure 6 Uptake by ES-2 cells.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Cur, curcumin; SH-ASA, SH-aspirin.
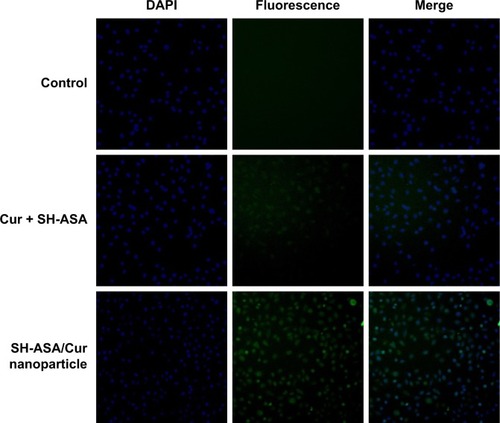
Figure 7 Anticancer abilities of free SH-ASA and Cur on ES-2 and SKOV3 human ovarian cancer cells in vitro.
Notes: (A) ES-2 cells; (B) SKOV3 cells.
Abbreviations: SH-ASA, SH-aspirin; Cur, curcumin.
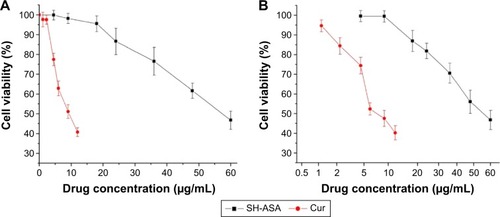
Figure 8 Anticancer effect of free SH-ASA and Cur combination.
Note: The results of the 1:4 and 1:6 combination groups demonstrated obvious synergistic effects.
Abbreviations: SH-ASA, SH-aspirin; Cur, curcumin.
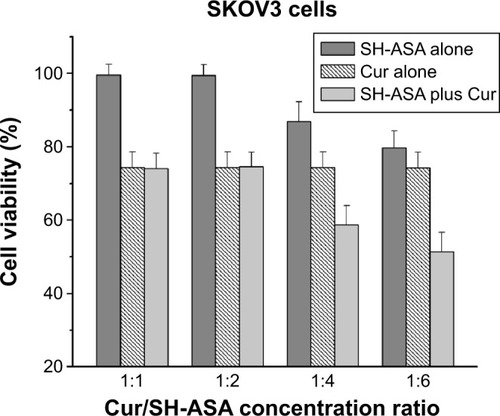
Figure 9 Anticancer abilities of different formulations containing SH-ASA and/or Cur against SKOV3 and ES-2 cells after treatment for 48 hours.
Notes: (A) SKOV3 cells; (B) ES-2 cells.
Abbreviations: SH-ASA, SH-aspirin; NPs, nanoparticles; Cur, curcumin.
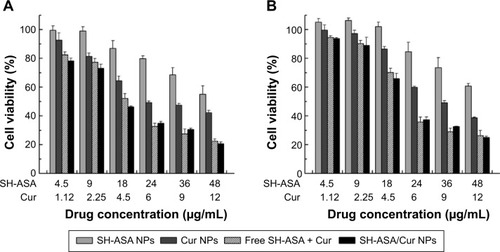
Figure 10 SH-ASA/Cur-coloaded mPEG-PLGA NPs induced apoptosis in human ovarian cancer cells.
Notes: (A) Control; (B) SH-ASA NPs; (C) Cur NPs; (D) SH-ASA/Cur NPs; (E) the early (Q4 region) and later (Q2 region) apoptosis rates of different formulations.
Abbreviations: FITC-A, Annexin V–fluorescein isothiocyanate; SH-ASA, SH-aspirin; NPs, nanoparticles; Cur, curcumin; mPEG-PLGA, methoxy poly(ethylene glycol)-poly (lactide-coglycolide).
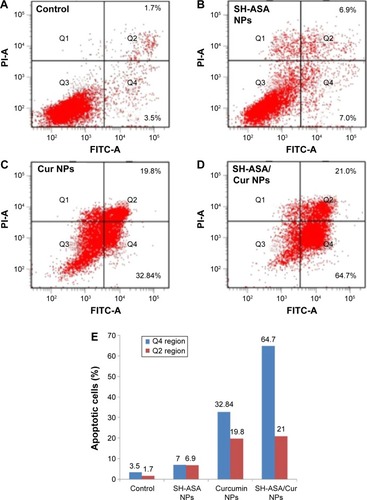
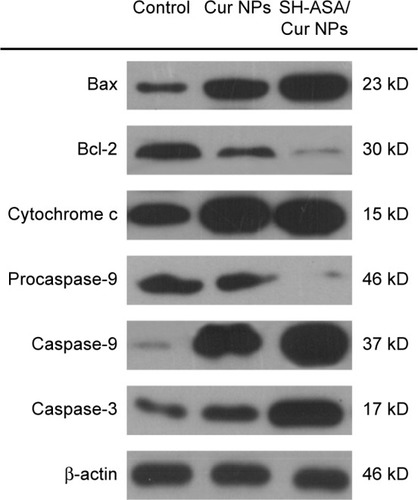
Figure 11 SH-ASA/Cur-codelivery mPEG-PLGA nanoparticles demonstrated a stronger effect when activating the mitochondrial-based apoptosis signaling pathway than did the single-drug formulation, suggesting that this might be a possible explanation for the synergistic anticancer effects.
Abbreviations: Cur, curcumin; NPs, nanoparticles; SH-ASA, SH-aspirin; mPEG-PLGA, methoxy poly(ethylene glycol)-poly (lactide-coglycolide).