Figures & data
Figure 1 Illustration of PLA–PEG copolymer synthesis (A) and OX26-modified AMB-loaded NPs synthesis (B).
Abbreviations: AMB, amphotericin B; EDC, ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; NHS, N-hydroxysuccinimide; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; PEG, poly(ethylene glycol); PLA, poly(d,l-lactide); TfR, transferrin receptor.
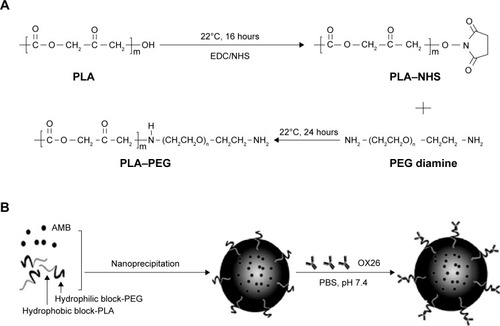
Figure 2 On day 6 after inoculation, (A) the levels of AMB in serum and brain were measured by HPLC, (B) shows the ratio of concentrations of AMB in brain to serum.
Notes: Each group included five animals. Data are expressed as the mean ± SEM.
Abbreviations: AMB, amphotericin B; HPLC, high-performance liquid chromatography; NP, nanoparticles; OX26, TfR monoclonal antibody of rats; SEM, standard error of mean; TfR, transferrin receptor.
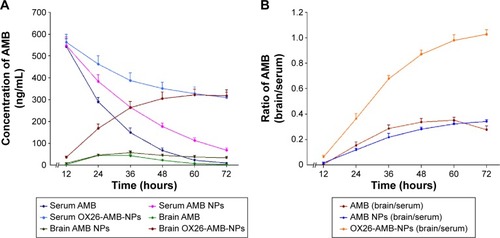
Figure 3 Characterization of PLA–PEG copolymer.
Notes: (A) TEM images of AMB NPs and OX26-AMB-NPs. (B) 1H NMR spectrum of PLA–PEG (500 MHz, CDCl3). The characteristic peaks of PLA ([a] CH:5.2 ppm and [c] CH3:1.6 ppm) and PEG ([b] CH2:3.6 ppm). (C) FTIR spectrum of PLA–PEG. Approximately 0.20 mg of the dry sample was mixed with IR-grade KBr (0.12 g) and pressed (10 ton) into tablet form. (D) DLS analysis of OX26-AMB-NPs and AMB NPs in water (0.020 mg/mL).
Abbreviations: AMB, amphotericin B; DLS, dynamic light scattering; FTIR, Fourier transform infrared spectroscopy; 1H NMR, proton nuclear magnetic resonance; IR, infrared; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PEG, polyethylene glycol; PLA, poly(lactic acid); TEM, transmission electron microscope; TfR, transferrin receptor.
![Figure 3 Characterization of PLA–PEG copolymer.Notes: (A) TEM images of AMB NPs and OX26-AMB-NPs. (B) 1H NMR spectrum of PLA–PEG (500 MHz, CDCl3). The characteristic peaks of PLA ([a] CH:5.2 ppm and [c] CH3:1.6 ppm) and PEG ([b] CH2:3.6 ppm). (C) FTIR spectrum of PLA–PEG. Approximately 0.20 mg of the dry sample was mixed with IR-grade KBr (0.12 g) and pressed (10 ton) into tablet form. (D) DLS analysis of OX26-AMB-NPs and AMB NPs in water (0.020 mg/mL).Abbreviations: AMB, amphotericin B; DLS, dynamic light scattering; FTIR, Fourier transform infrared spectroscopy; 1H NMR, proton nuclear magnetic resonance; IR, infrared; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PEG, polyethylene glycol; PLA, poly(lactic acid); TEM, transmission electron microscope; TfR, transferrin receptor.](/cms/asset/32896756-fd48-4cb1-8326-41f340455be5/dijn_a_84656_f0003_c.jpg)
Table 1 The particle size, PDI, zeta potential, and EF% of the PLA–TPGS copolymer (n=3)
Figure 4 Cumulative release profiles of AMB-NPs and OX26-AMB-NPs in PBS (pH =7.35) at 37°C.
Abbreviations: AMB, amphotericin B; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; PEG, polyethylene glycol; PLA, poly(lactic acid); TfR, transferrin receptor.
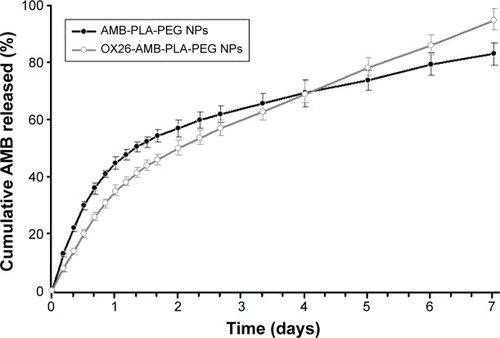
Figure 5 In vitro cytotoxicities of AMB-loaded formulations.
Notes: (A) Hemolysis ratio (%) of free AMB and AMB-loaded formulations. (B) Cytotoxicity of free AMB and AMB-loaded formulations against CCC-HEK-1 cell line with the MTT assay. Data from three independent experiments were expressed as mean ± SEM (n=3).
Abbreviations: AMB, amphotericin B; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; PEG, polyethylene glycol; PLA, poly(lactic acid); SEM, standard error of mean; TfR, transferrin receptor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
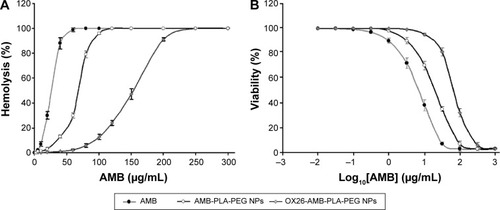
Figure 6 Nephrotoxicity assessment after different AMB formulation treatments.
Notes: Normal control rats show no abnormality and no KIM-1 or NGAL expression. AMB and AMB NP treatments show high-level expression of KIM-1 and NGAL, but OX26-AMB-NP treatment shows no KIM-1 or NGAL expression. All groups of mice show no expression of apoptosis marker TUNEL under AMB therapeutic concentration (×200).
Abbreviations: AMB, amphotericin B; H&E, hematoxylin and eosin stain; IHC, immunohistochemistry; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; TfR, transferrin receptor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay.
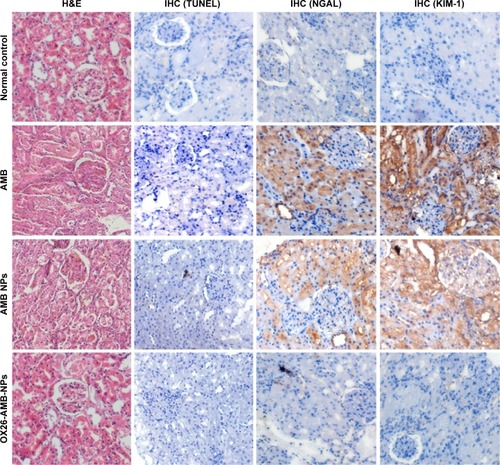
Table 2 Effects of different drug-induced release of ALT, GLDH, and GSTα
Figure 7 Using TUNEL assay in rats, hepatic histopathology and immunohistochemistry were used to detect cell inflammation and cell apoptosis.
Notes: PBS-treated normal control, showing normal lobular architecture and cell structure; AMB-treated group, showing extensive hepatic centrilobular damage characterized by the presence of vacuolization and severe apoptosis; AMB NPs-treated group, showing modest hepatic centrilobular degeneration and apoptosis; OX26-AMB-NPs, showing lobular architecture and cell structure similar to the normal control.
Abbreviations: AMB, amphotericin B; H&E, hematoxylin and eosin stain; IHC, immunohistochemistry; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; TfR, transferrin receptor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay.
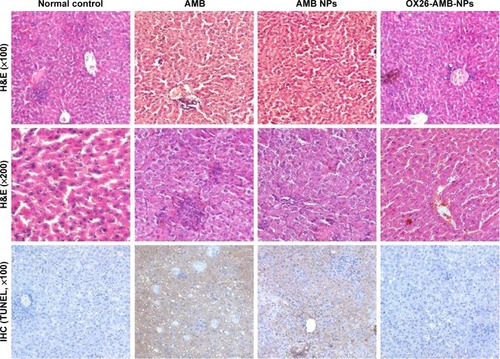
Figure 8 Toxicity assessment of the main tissues after different AMB formulation treatments.
Notes: Macroscopic appearance of histological H&E and apoptosis marker TUNEL immunohistochemical stains. Histopathological examination of the mice lung/heart/brain detected no obvious pathological changes or cell apoptosis after OX26-AMB-NP (equivalent free AMB, 1 mg/kg) treatment for 48 hours.
Abbreviations: AMB, amphotericin B; H&E, hematoxylin and eosin stain; IHC, immunohistochemistry; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; TfR, transferrin receptor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay.
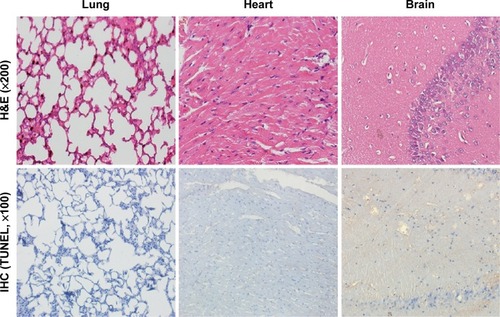
Figure 9 Cellular uptake of OX26-coumarin 6-NPs and surface levels of TfR.
Notes: (A) All the cells were dosed with 50 μg/mL fluorescein-labeled NPs for 4 hours at 37°C and collected for CLSM analysis. The OX26-coumarin 6-NPs were green (EGFP channel), and the nuclei were stained by DAPI (blue). The cellular uptake was visualized by overlaying images obtained using the EGFP filter and DAPI filter. A kinetic study of cell uptake was performed with three cell lines that expressed TfR at different levels: HeLa (TfRhigh) > SKOV3 (TfRhigh) > HEK 293 (TfRlow) (×200). (B) Surface TfR levels decreased after 24 hours OX26-coumarin 6-NP incubation at 50 μg/mL conditions as confirmed by Western blot in HeLa and SKOV3 cell lines. (C) Quantification of the remaining TfR fraction on the cell surface after 24 hours incubation with anti-TfR bispecifics. Mean ± SEM; *P<0.01 by one-tailed Student’s t-test for TfR/actin ratio.
Abbreviations: CLSM, confocal laser scanning microscopy; DAPI, 4′,6′-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; SEM, standard error of mean; TfR, transferrin receptor.
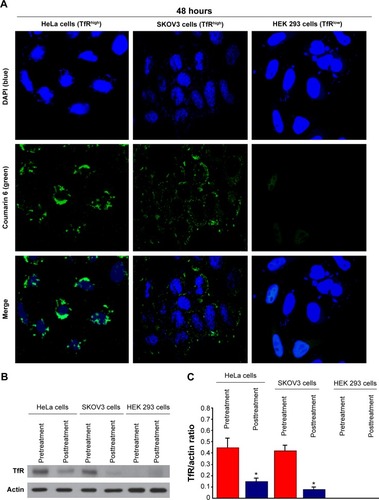
Figure 10 Photomicrographs of brain section showing the treatment efficacy of AMB formulations for Candida glabrata infection on day 15 after inoculation.
Notes: A histopathological analysis of H&E staining and Gram’s/Brilliant green staining for brain sections were performed. Groups: normal group, PBS group, null NPs group, AMB group, AMB NPs group, and OX26-AMB-NPs group.
Abbreviations: AMB, amphotericin B; H&E, hematoxylin and eosin stain; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; TfR, transferrin receptor.
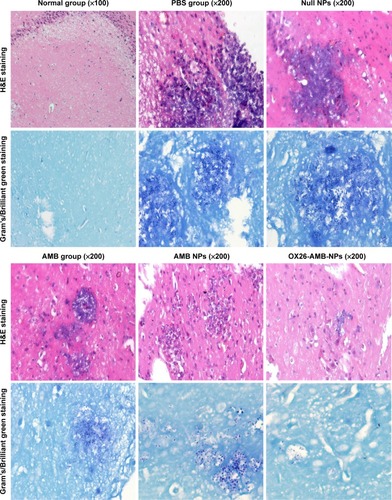
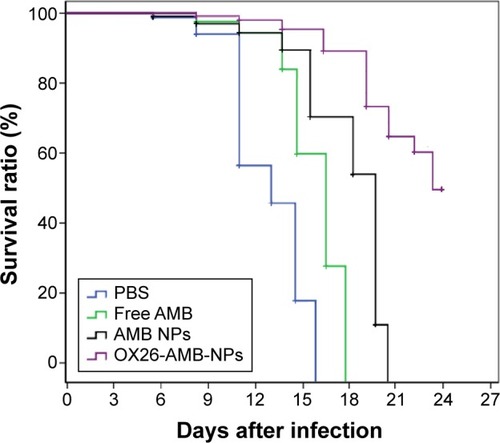
Figure 11 Survival curves of BALB/c mice intracranially infected with Candida glabrata 105 CFU/animal and treated with free AMB, AMB NPs, and OX26-AMB-NPs at 3 mg/kg from day 2 to 4 after infection.
Abbreviations: AMB, amphotericin B; CFU, colony-forming unit; NP, nanoparticle; OX26, TfR monoclonal antibody of rats; PBS, phosphate-buffered saline; TfR, transferrin receptor.