Figures & data
Figure 1 Synthesis of X-shaped amphiphilic copolymers.
Abbreviations: PLGA-COOH, poly(lactic-co-glycolic acid); SS, disulfide; PEG, poly(ethylene glycol).
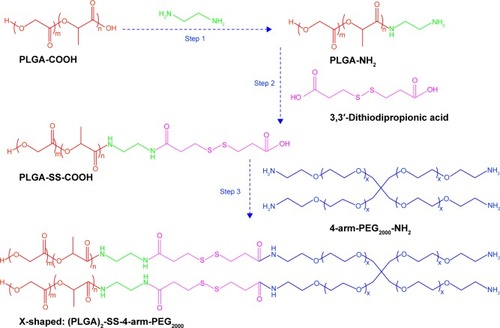
Figure 2 Synthesis scheme for amphiphilic copolymers with different shapes and associated 1H-NMR spectra (400 MHz, DMSO-d6 as solvent).
Notes: Polymer characterization: 1H-NMR: A (δ 4.93, s, (–OC H2COO–)), B (δ 5.22, q, (–OC H(CH3)CONH–)), C (δ 1.50, t, (–OCH(C H3)CONH–)), D (δ 3.19, t, (–NH CH2CH2NH–)), G (δ 2.61, s, (–C H2-SS- CH2–)), J (δ 3.52, t, (–CH2C H2O–)).
Abbreviations: PLGA-COOH, poly(lactic-co-glycolic acid); SS, disulfide; PEG, poly(ethylene glycol); ppm, parts per million; 1H-NMR, 1H-nuclear magnetic resonance imaging.
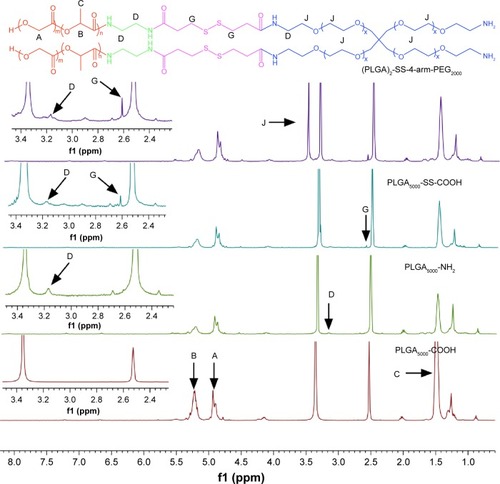
Figure 3 FTIR spectra for (A) PLGA-COOH, (B) PLGA-NH2, (C) PLGA-SS-COOH, (D) (PLGA)2-SS-4-arm-PEG2000-NH2.
Abbreviations: PLGA-COOH, poly(lactic-co-glycolic acid); SS, disulfide; PEG, poly(ethylene glycol); FTIR, Fourier transform infrared spectroscopy.
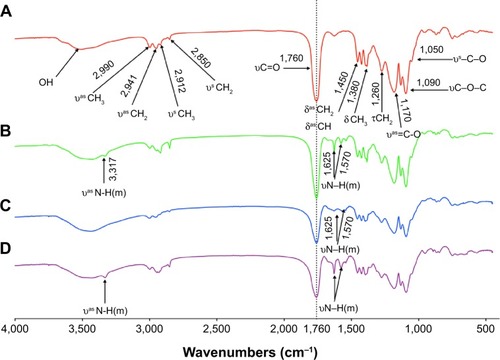
Table 1 Summary of GPC results for the synthesized block copolymers
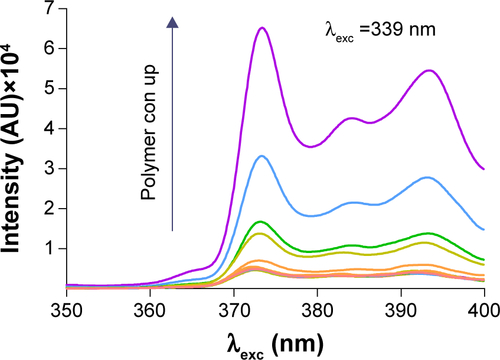
Figure 4 Determination of Micelle Formation.
Notes: (A) The emission fluorescence intensity of coumarin 6 in water. (B) The emission fluorescence of coumarin 6 (20 µg/mL) in acetone and nanomicelles. (C) Fluorescence quenching process of coumarin 6 in acetone caused by the addition of water. (D) SEM image of the formed particles.
Abbreviations: XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; SEM, scanning electron microscopy; PLGA, poly(lactic-co-glycolic acid); PEG, poly (ethylene glycol).
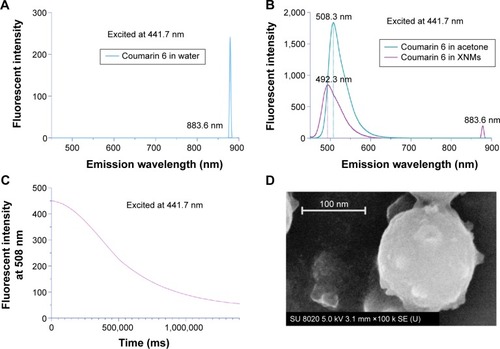
Figure 5 Comparison of Micellar Stability.
Notes: (A) Plot of fluorescence intensity ratio against the logarithm of polymer concentration. (Fluorescence emission spectra of pyrene are shown in ). (B) Size changes of nanomicelles. (The original data are shown in ).
Abbreviations: XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; LNMs, loaded linear (PLGA)2-SS-4-arm-PEG200 polymer nanomicelles; PDI, polydispersity index; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol).
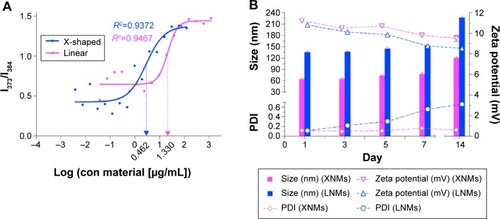
Figure 6 (A) Typical DLS images of XNMs without DTT. (B) The DLS images of XNMs suspensions with DTT (10 mmol/L) for up to 24 hours.
Abbreviations: DLS, dynamic light scattering; XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; LNMs, loaded linear (PLGA)2-SS-4-arm-PEG200 polymer nanomicelles; DTT, DL-Dithiothreitol; PDI, polydispersity index; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol).
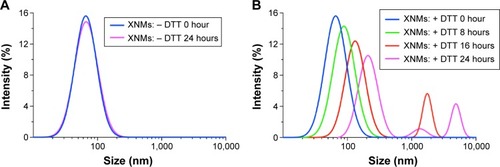
Table 2 Some characteristics of DTX-loaded nanomicelles
Figure 7 (A) Typical SEM images of DTX/XNMs (insert: typical appearance of DTX/XNMs suspension). (B) Typical TEM images of DTX/XNMs. (C) In vitro drug release profile of DTX/XNMs. (D) Changes of particle size, PDI, and drug remaining percentage of DTX/XNMs in process of time.
Abbreviations: DTX, docetaxel; XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; TEM, transmission electron microscopy; PDI, polydispersity index; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol).
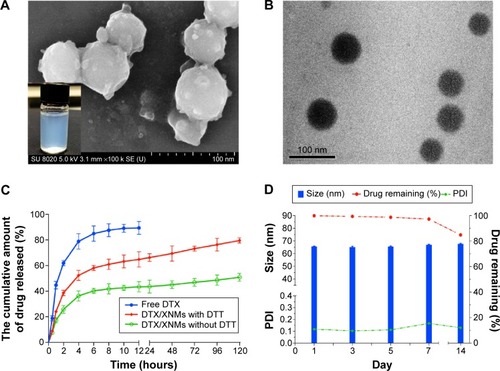
Figure 8 (A) The column graph showing cellular coumarin 6 fluorescence in A2780 cells following certain time incubation. Indicated values were mean ± SD (n=6). *P<0.05, **P<0.01, ***P<0.001. (B) Cellular uptake of coumarin 6, coumarin 6/LNMs, and coumarin 6/XNMs analyzed by flow cytometry.
Abbreviations: XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; LNMs, loaded linear (PLGA)2-SS-4-arm-PEG200 polymer nanomicelles; FITC, fluorescein isothiocyanate; SD, standard deviation; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol).
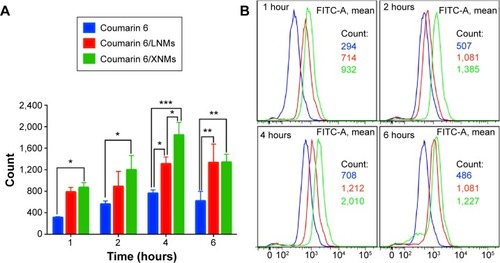
Figure 9 The in vitro cytotoxicity assessment of XNMs and drug loaded formulations using MTT assay.
Notes: (A) Biocompatibility assessment of XNMs against A2780 cells following incubation for 48 hours. (B) Cytotoxicity effect of DTX and DTX loaded formulations on A2780 after treatment for 24 hours. (C) Cytotoxicity effect of DTX and DTX loaded formulations on A2780 after 48 hours incubation. Indicated values were mean ± SD (n=6). *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: DTX, docetaxel; XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol); LNMs, loaded linear (PLGA)2-SS-4-arm-PEG200 polymer nanomicelles.
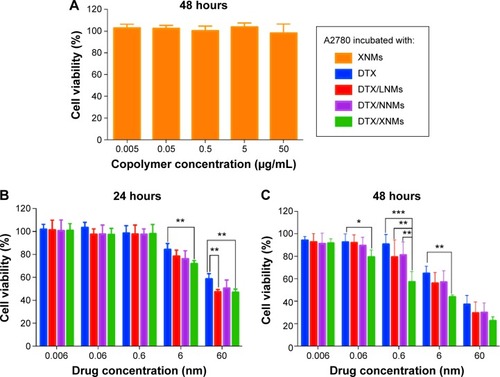
Figure 10 (A) Immunofluorescence images for nuclear staining (Hoechst33342: blue) and β-tubulin (FITC: green) following the treatment with different DTX formulations for 24 hours. (B) Fluorescence imaging analysis of apoptosis in A2780 cells.
Abbreviations: DTX, docetaxel; XNMs, X-shaped (PLGA)2-SS-4-arm-PEG2000 polymer nanomicelles; LNMs, loaded linear (PLGA)2-SS-4-arm-PEG200 polymer nanomicelles; FITC, fluorescein isothiocyanate; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol).
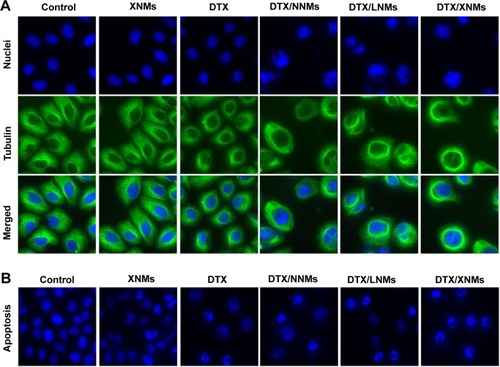
Figure S1 Typical GPC chromatograms of polymers (A) PLGA-NH2 (B) PLGA-SS-COOH.
Abbreviations: GPC, gel permeation chromatography; PLGA, poly(lactic-co-glycolic acid); SS, disulfide; MV, micro voltage.
Figure S2 Typical GPC chromatograms of three synthesized amphiphilic block copolymers.
Note: –SS– represents the disulphide bonds.
Abbreviations: GPC, gel permeation chromatography; PLGA, poly(lactic-co-glycolic acid); MV, micro voltage.
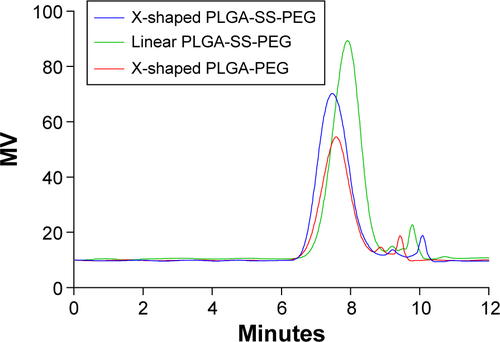
Table S1 Summary of GPC results
Table S2 Changes of size, PDI, and zeta potential of XNMs and LNMs