Figures & data
Table 1 Composition of PTX-loaded SLNs or PTX-loaded SLNs modified with HPCD
Figure 1 Cell proliferation of PTX-loaded SLNs (A) and PTX-loaded SLNs modified with HPCD (B) at 5 µM or 10 µM PTX concentration for 24 hours, 48 hours, or 72 hours incubation by MTT assay (n=3, mean ± SD). *P<0.05; **P<0.01.
Abbreviations: PTX, Paclitaxel; SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.
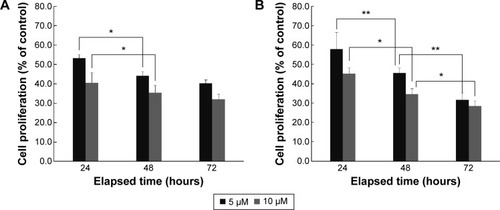
Figure 2 Cellular uptake into MCF-7 cells treated with Nile red solution, Nile red-loaded SLNs, or Nile red-loaded SLNs modified with HPCD for 2 hours or 8 hours of incubation.
Notes: (A) Nile red solution for 2 hours, (B) Nile red solution for 8 hours, (C) Nile red-loaded SLNs for 2 hours, (D) Nile red-loaded SLNs for 8 hours, (E) Nile red-loaded SLNs modified with HPCD for 2 hours, (F) Nile red-loaded SLNs modified with HPCD for 8 hours.
Abbreviations: SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.
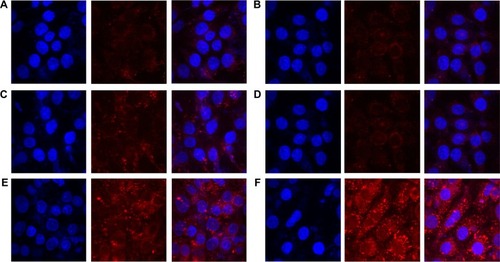
Figure 3 Apoptotic assay in MCF-7 cells treated with PTX solution, PTX-loaded SLNs, or PTX-loaded SLNs modified with HPCD corresponding 10 µM of PTX for 2 hours or 8 hours of incubation.
Notes: (A) PTX solution for 2 hours, (B) PTX solution for 8 hours, (C) PTX-loaded SLNs for 2 hours; (D) PTX-loaded SLNs for 8 hours, (E) PTX-loaded SLNs modified with HPCD for 2 hours, (F) PTX-loaded SLNs modified with HPCD for 8 hours.
Abbreviations: PTX, Paclitaxel; SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.
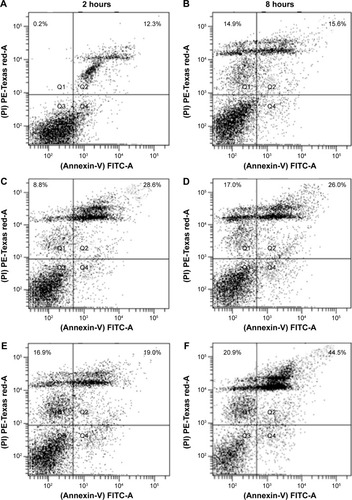
Table 2 The pharmacokinetic parameters observed in rats after intravenous injection of 5 mg/kg dose of PTX solution, PTX-loaded SLNs or PTX-loaded SLNs modified with HPCD. The results represent the mean ± SD of three rats
Figure 4 The concentration of PTX levels in rat plasma after intravenous injection of 5 mg/kg dose of PTX solution, PTX-loaded SLNs (PS) or PTX-loaded SLNs modified with HPCD (PSC).
Note: Each point represents the mean ± SD of three rats per time point.
Abbreviations: PTX, Paclitaxel; SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.
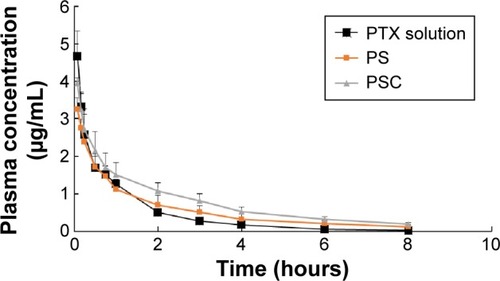
Figure 5 The normalized kidney weight (A) and serum creatinine level (B) of intravenous injection with PTX solution, PTX-loaded SLNs (PS), or PTX-loaded SLNs modified with HPCD (PSC) after 8 hours of intravenous injection
Notes: n=3, mean ± SD. *P<0.05 compared with control.
Abbreviations: PTX, Paclitaxel; SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.
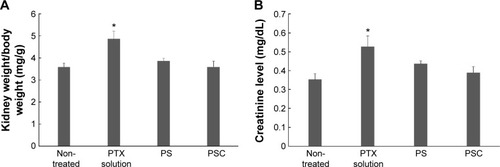
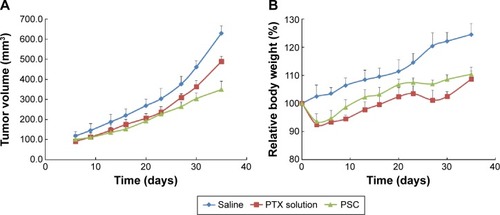
Figure 6 The effect of PTX-loaded SLNs modified with HPCD on tumor volume and body weight.
Notes: The changes of tumor volumes (A) and body weight (B) according to the administration of saline, PTX solution, or PTX-loaded SLNs modified with HPCD (PSC) (n=3, mean ± SD).
Abbreviations: PTX, Paclitaxel; SLNs, solid lipid nanoparticles; HPCD, 2-hydroxypropyl-β-cyclodextrin.