Figures & data
Figure 1 A) Lipid BF of dioctadecyldimethylammonium bromide (DODAB)Citation45 or B) sodium dihexadecylphosphate (DHP)Citation27 or C) DSPC/cholesterol/PEG-DSPE(5000) mixtures at 12 mol% PEG-DSPE(5000)Citation42 or D) DSPC: cholesterol: ceramide-PEG5000 carrying bacteriorhodopsin.Citation43
Notes: With exception of micrograph in B) which was obtained by TEM after negatively staining the sample, all micrographs were obtained by cryo-TEM. In C), disks were observed edge-on (arrow) or face-on (arrow head). Bars denote 100 nm. Copyright © 1995 and 1991 American Chemical Society; © 2005 and 2007 Elsevier. Adapted with permission from Carmona-Ribeiro AM, Castuma CE, Sesso A, Schreier S. Bilayer structure and stability in dihexadecyl phosphate dispersions. J Phys Chem. 1991;95:5361–5366. Johansson E, Engvall C, Arfvidsson M, Lundahl P, Edwards K. Development and initial evaluation of PEG-stabilized bilayer disks as novel model membranes. Biophys Chem. 2005;113:183–192. Johansson E, Lundquist A, Zuo S, Edwards K. Nanosized bilayer disks: attractive model membranes for drug partition studies. Biochim Biophys Acta. 2007;1768:1518–1525. Andersson M, Hammarstrom L, Edwards K. Effect of bilayer phase transitions on vesicle structure, and its influence on the kinetics of viologen reduction. J Phys Chem. 1995;99(39):14531–14538.
Abbreviations: DHP, sodium dihexadecylphosphate; DODAB, dioctadecyldimethylammonium bromide; DSPE, distearoylphosphatidylethanolamine; PEG, polyethyleneglycol; TEM, transmission electron microscopy.
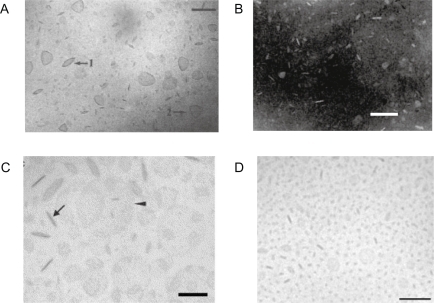
Figure 2 The interaction between one bilayer vesicle and two particles. Citation6,Citation7, Citation53–Citation61 Copyright © 1999 Elsevier. Adapted with permission from Carmona-Ribeiro AM, Lessa MM. Interactions between bilayer vesicles and latex. Colloids Surf A. 1999;153:355–361.
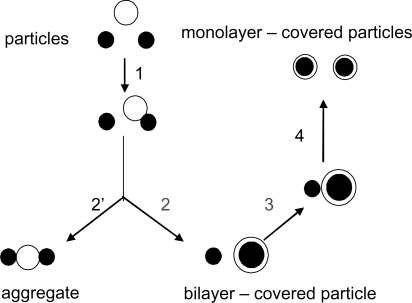
Table 1 Differential cytotoxicity of DODAB against some eukaryotic* and prokaryotic cells**
Figure 3 Superior performance of novel DODAB-based adjuvants inducing DTH in mice as compared to alum. The same antigen (Ag) carried by each adjuvant was used for immunization. Ag was carried by DODAB BF at 0.1 mM DODAB (DODAB BF/Ag) or by PSS/DODAB or silica/DODAB particles at 0.01 or 0.05 mM DODAB (PSS/DODAB/Ag or silica/DODAB/Ag), respectively, or by alum (Al(OH)Citation3/Ag). After immunization, elicitation of the swelling response was done by injecting Ag alone in the footpad so that % footpad swelling was measured in comparison to alum. Copyright © 2007, 2009 Elsevier. Adapted with permission from Lincopan N, Espíndola NM, Vaz AJ, Carmona-Ribeiro AM. Cationic supported lipid bilayers for antigen presentation. In. J Pharm. 2007;340:216–222. Lincopan N, Espíndola NM, Vaz AJ, et al. Novel immunoadjuvants based on cationic lipid: preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–5771. Lincopan N, Santana MRA, Faquim-Mauro E, da Costa MHB, Carmona-Ribeiro AM. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009;9:article 5.
Abbreviations: DODAB, dioctadecyldimethylammonium bromide; PSS, polystyrene sulfate; DTH, delayed-type hypersensibility; BF, bilayer fragments.
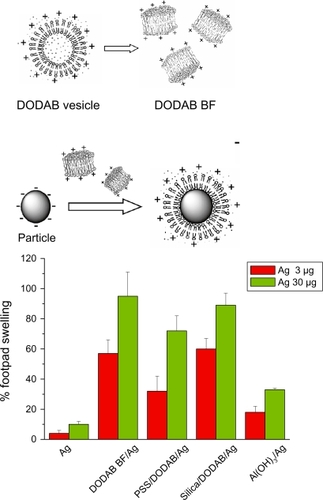
Table 2 Physical properties of the novel cationic immunoadjuvants in a1 mM NaCl (pH 6.3) or b5 mM TrisHCl (pH 7.4) at 5 × 109 PSS particles/ml or 0.1 mg/ml silica or 0.1 mg/ml Al(OH)3. Copyright © 2009 Elsevier. Adapted with permission from Lincopan N, Espíndola NM, Vaz AJ, et al. Novel immunoadjuvants based on cationic lipid: preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–5771.
Table 3 Minimal fungicidal concentration (MFC) for Zoltec® (fluconazol), miconazole (MCZ), MCZ/DODAB BF or MCZ/DHP BF against Candida albicans. Copyright © 2006 Elsevier. Adapted with permission from Vieira DB, Pacheco LF, Carmona-Ribeiro AM. Assembly of a model hydrophobic drug into cationic bilayer fragments. J Colloid Interface Sci. 2006;293:240–247.
Figure 4 A) Encapsulation of amphotericin B particle by a cationic bilayer at high drug to lipid molar ratio; B) Solubilization of amphotericin B at the rim of cationic BF at low drug to lipid molar ratio.

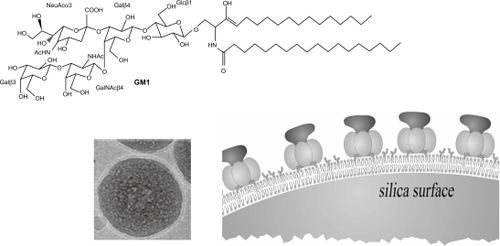
Figure 5 Receptor-ligand recognition on biomimetic particles.Citation60 Cryo-TEM revealed the PC bilayer surrounding a silica particle. The GM1 receptor inserted in supported PC bilayers recognized its ligand, the cholera toxin. Copyright © 2005 American Chemical Society. Adapted with permission from Mornet S, Lambert O, Duguet E, Brisson A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005;5:281–285
Abbreviations: TEM, transmission electron microscopy; PC, phosphatidylcholine.