Figures & data
Table 1 Particle sizes and zeta potentials of different liposomes (n=3, mean ± SD)
Figure 1 Transmission electron microscope (TEM) images and diameter profiles of the optimized CL (A), Sf-CL (B), SiSf-CL (C), CMCS-SiSf-CL (D).
Abbreviations: CL, blank cationic liposomes; Sf-CL, sorafenib-loaded cationic liposomes; SiSf-CL, siRNA and sorafenib co-delivery cationic liposomes; CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes.
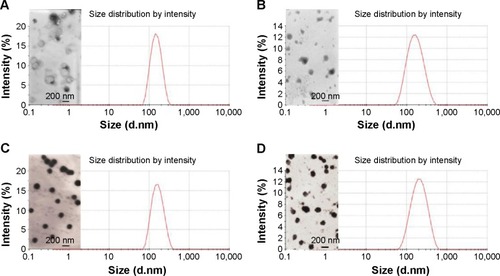
Figure 2 Agarose gel electrophoresis, particle size, and zeta potential of SiSf-CL and CMCS-SiSf-CL.
Notes: Complexation of siRNA with Sf-CL in agarose gel at various CL/siRNA ratios (A), and then further complexing with CMCS at various CMCS/siRNA ratios (CL/siRNA =20) (B). Particle sizes and zeta potentials of SiSf-CL formed at different CL/siRNA ratios without CMCS coating (C). Particle sizes and zeta potentials of SiSf-CL (CL/siRNA =20) with CMCS coating at various CMCS/siRNA ratios (D). Data are mean ± SD (n=3).
Abbreviations: CL, blank cationic liposomes; CMCS, carboxymethyl chitosan; CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; Sf-CL, sorafenib-loaded cationic liposomes; SiSf-CL, siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA; SD, standard deviation.
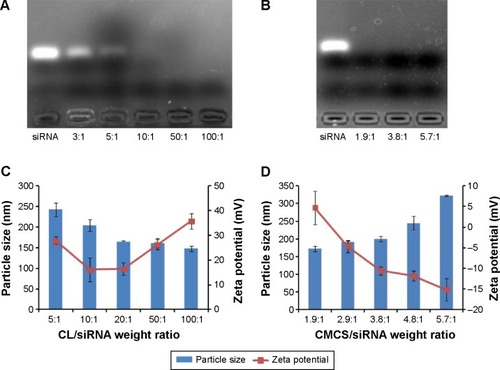
Figure 3 In vitro drug release profiles of Sf from CMCS-Sf-CL and CMCS-SiSf-CL in pH 6.5 and 7.4 medium at 37°C.
Note: Data are mean ± SD (n=3).
Abbreviations: CMCS-Sf-CL, carboxymethyl chitosan-modified sorafenib-loaded cationic liposomes; CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA.
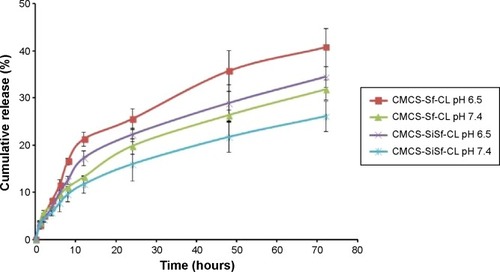
Figure 4 Stability of naked siRNA and CMCS-SiSf-CL after treatment with serum (A) or RNase (B) for different times.
Abbreviations: CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA; small interfering RNA; h, hours.

Figure 5 In vitro uptake study.
Notes: Fluorescent micrographs (A), flow cytometric histogram profiles of fluorescence intensity (B and C) of SiSi-CL, CMCS-SiSf-CL at pH 7.4 and CMCS-SiSf-CL at pH 6.5 in HepG2 cells following 4 hours of incubation at 37°C, with free siRNA as negative control and Lipofectamine-2000 as positive control. Data are mean ± SD (n=3). *P<0.05.
Abbreviations: Lipo 2000, Lipofectamine-2000; SiSf-CL, siRNA and sorafenib co-delivery cationic liposomes; CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA.
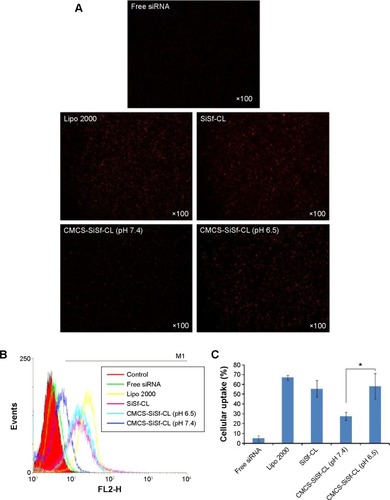
Table 2 IC50 of various liposomal formulations against HepG2 cells following 24-hour incubation (n=3)
Figure 6 In vitro cytotoxicities of various liposomal formulations against HepG2 cells following 24-hour incubation.
Notes: Data are mean ± SD (n=3). *P<0.05.
Abbreviations: Sf, sorafenib; CL, blank cationic liposomes; Sf-CL, sorafenib-loaded cationic liposomes; SiSf-CL, siRNA and sorafenib co-delivery cationic liposomes; CMCS-Sf-CL, carboxymethyl chitosan-modified sorafenib-loaded cationic liposomes; CMCS-CL, carboxymethyl chitosan-modified blank cationic liposomes; CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA.
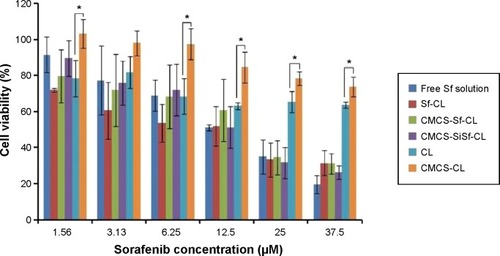
Figure 7 IVIS images of tumor accumulation.
Notes: In vivo CMCS-SiSf-CL distribution in female Kunming mice after intravenous injection at 6 hours with free Cy5-siRNA or CMCS-SiSf-CL made of Cy5-siRNA. Arrows indicate H22 tumors.
Abbreviations: CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA; IVIS, in vivo real-time fluorescence imaging system.
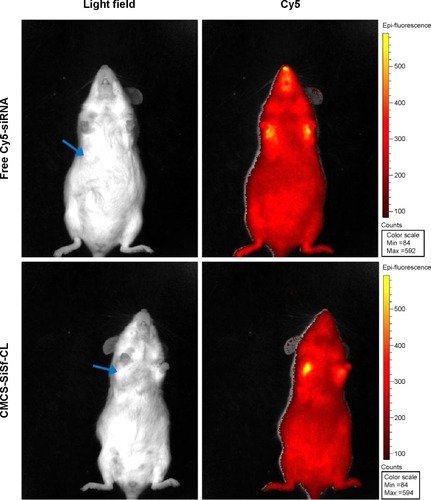
Figure 8 In vivo uptake study.
Notes: Tumors from sacrificed mice were collected, frozen, and sectioned. Sections were counterstained with Hoechst 33342 and visualized with fluorescence microscope.
Abbreviations: CMCS-SiSf-CL, carboxymethyl chitosan-modified siRNA and sorafenib co-delivery cationic liposomes; siRNA, small interfering RNA.
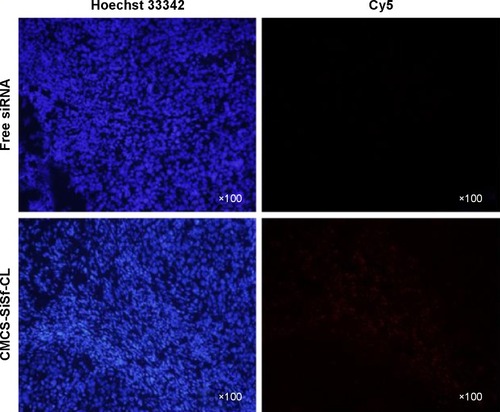
Figure 9 In vivo antitumor efficacy study in H22 cells-bearing Kunming mice tumor model after intravenous injection of PBS, free Sf solution, CL, Sf-CL, CMCS-Sf-CL, and CMCS-CL.
Notes: (A) Relative tumor volume; (B) average tumor mass isolated from the mice of each experimental group; (C) variation in body weight as a function of time. Data are mean ± SD (n=5). **P<0.01, *P<0.05.
Abbreviations: Sf, sorafenib; CL, blank cationic liposomes; Sf-CL, sorafenib-loaded cationic liposomes; CMCS-Sf-CL, carboxymethyl chitosan-modified sorafenib-loaded cationic liposomes; CMCS-CL, carboxymethyl chitosan-modified blank cationic liposomes; PBS, phosphate-buffered saline; siRNA, small interfering RNA.
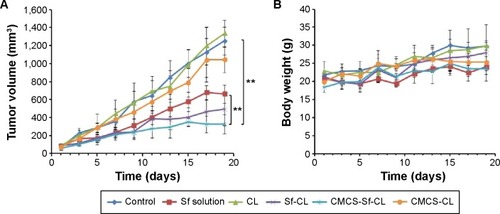
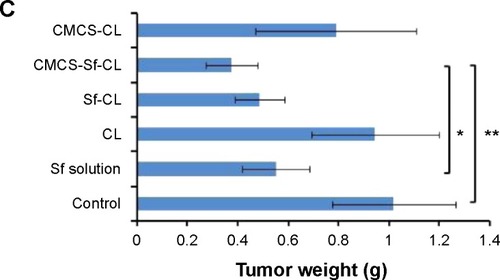
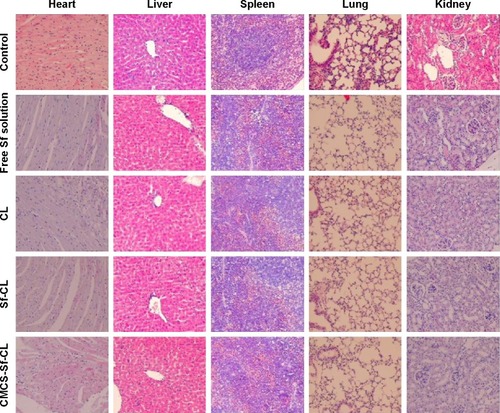
Figure 10 Evaluation of systemic toxicities by H&E staining showing histopathological changes in the major visceral organs.
Abbreviations: Sf, sorafenib; CL, blank cationic liposomes; Sf-CL, sorafenib-loaded cationic liposomes; CMCS-Sf-CL, carboxymethyl chitosan-modified sorafenib-loaded cationic liposomes; H&E, hematoxylin and eosin.