Figures & data
Table 1 Composition of SMSDs
Figure 1 Aqueous solubility of the drug in various carriers: (A) oils, (B) 1% surfactants in an aqueous solution, and (C) 0.1% of polymers in an aqueous solution.
Note: Each value represents the mean ± SD (n=3).
Abbreviations: GMC, glyceryl monocaprylate; SLS, sodium lauryl sulfate; HPMC, hydroxypropylmethylcellulose; HPC, hydroxypropylcellulose; PVP, poly(vinyl pyrrolidone); PVA, polyvinyl alcohol; Na CMC, sodium carboxymethyl cellulose; SD, standard deviation.
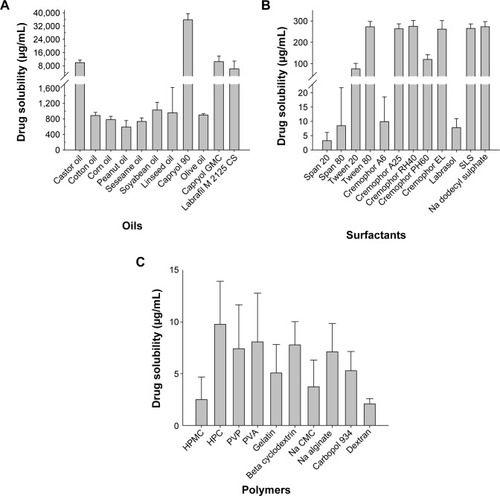
Figure 2 Pseudoternary phase diagram using Capryol 90 as an oil, Cremophor EL as a surfactant, and Tween 80 as a cosurfactant.
Note: The blackened area is microemulsion area.
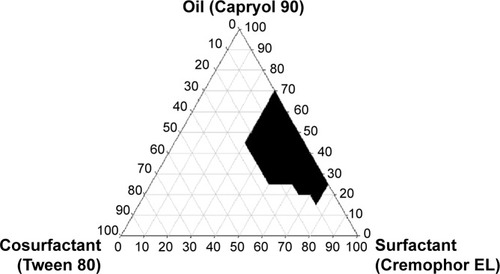
Figure 3 Effect of carriers on the emulsion droplet size of liquid SNEDDS: (A) effect of the ratio of surfactant to the oil on the droplet size of the emulsion formed from an oil/surfactant mixture and (B) effect of the cosurfactant percentage volume ratio on the mean emulsion droplet diameter of formulations containing 20% of constant surfactant volume.
Note: Each value represents the mean ± SD (n=3).
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SD, standard deviation.
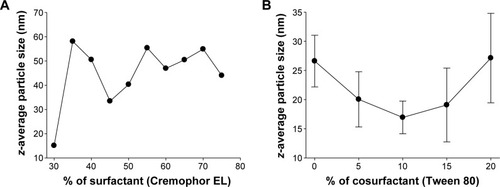
Figure 4 Aqueous drug solubility in various SMSDs.
Notes: The compositions of SMSDs are shown in . Each value represents the mean ± SD (n=3). *P<0.05 when compared with the drug powder and SM1. **P<0.05 when compared with the SM2 and SM3. ***P<0.05 when compared with the SM4.
Abbreviations: SMSDs, surface modified solid dispersions; SD, standard deviation.
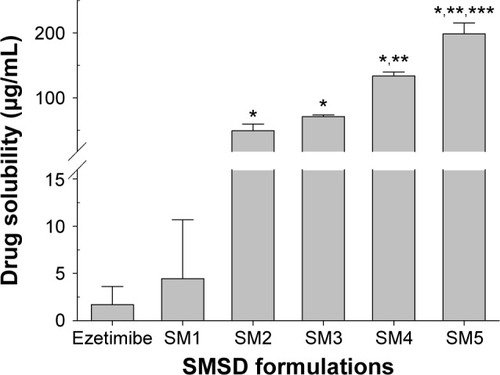
Figure 5 Scanning electron micrographs: (A) drug powder (50,000×), (B) solid SNEDDS (5,000×), (C) SMSD (10,000×), and (D) SESD (5,000×).
Notes: The solid SNEDDS was composed of silicon dioxide and liquid SNEDDS (1.5:3, w/v), which consisted of ezetimibe/Capryol 90/Cremophor EL/Tween 80 at a ratio of 5:10:35:55 (w/v/v/v). The SMSD or SESD was composed of the drug, HPC, and Tween 80 at a weight ratio of 3/1.5/1.5.
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion; HPC, hydroxypropylcellulose.
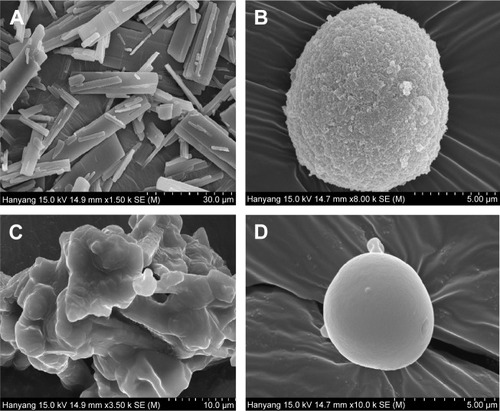
Figure 6 DSC (A), PXRD (B), and FTIR (C): (a) drug powder, (b) silicon dioxide, (c) physical mixture of ezetimibe and silicon dioxide, (d) solid SNEDDS, (e) HPC, (f) physical mixture of ezetimibe and HPC, (g) SMSD, and (h) SESD.
Notes: The solid SNEDDS was composed of silicon dioxide and liquid SNEDDS (1.5:3, w/v), which consisted of ezetimibe/Capryol 90/Cremophor EL/Tween 80 at a ratio of 5:10:35:55 (w/v/v/v). The SMSD or SESD was composed of the drug, HPC, and Tween 80 at a weight ratio of 3/1.5/1.5.
Abbreviations: DSC, differential scanning calorimetry; PXRD, powder x-ray diffraction; FTIR, Fourier-transform infrared spectroscopy; SNEDDS, self-nanoemulsifying drug delivery system; HPC, hydroxypropylcellulose; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion.
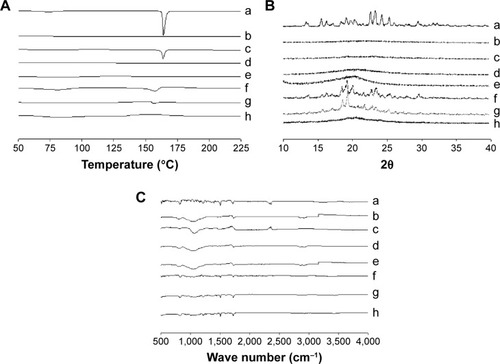
Figure 7 Cumulative undersize percentage of solid SNEDDS, SMSD, and SESD.
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion.
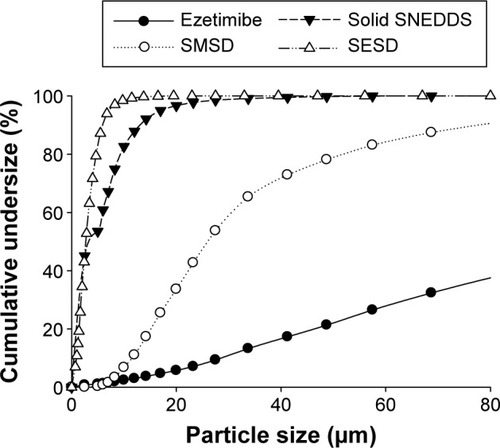
Figure 8 Aqueous drug solubility in solid SNEDDS, SMSD, and SESD: (A) water, (B) pH 1.2, (C) pH 4.0, and (D) pH 6.8.
Notes: Each value represents the mean ± SD (n=3). The solid SNEDDS was composed of silicon dioxide and liquid SNEDDS (1.5:3, w/v), which consisted of ezetimibe/Capryol 90/Cremophor EL/Tween 80 at a ratio of 5:10:35:55 (w/v/v/v). The SMSD or SESD was composed of the drug, HPC, and Tween 80 at a weight ratio of 3/1.5/1.5. *P<0.05, **P<0.05, and ***P<0.05 when compared with the drug powder, SMSD, and SESD, respectively.
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion; SD, standard deviation; HPC, hydroxypropylcellulose.
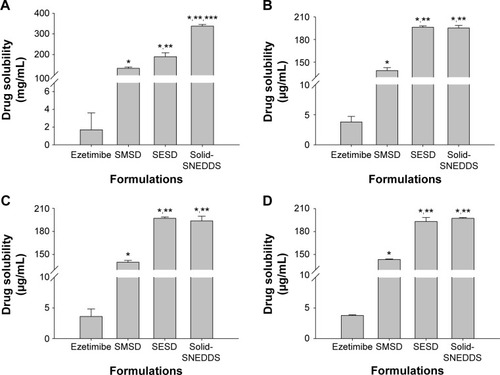
Figure 9 Dissolution profile of the drug from solid SNEDDS, SMSD, and SESD: (A) water, (B) pH 1.2, (C) pH 4.0, and (D) pH 6.8.
Notes: Each value represents the mean ± SD (n=6). The solid SNEDDS was composed of silicon dioxide and liquid SNEDDS (1.5:3, w/v), which consisted of ezetimibe/Capryol 90/Cremophor EL/Tween 80 at a ratio of 5:10:35:55 (w/v/v/v). The SMSD or SESD was composed of the drug, HPC, and Tween 80 at a weight ratio of 3/1.5/1.5. *P<0.05, **P<0.05, and ***P<0.05 when compared with the drug powder, SMSD, and SESD, respectively.
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion; SD, standard deviation; HPC, hydroxypropylcellulose.
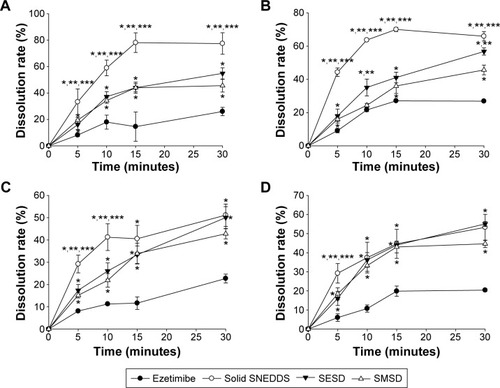
Table 2 Pharmacokinetic parameters
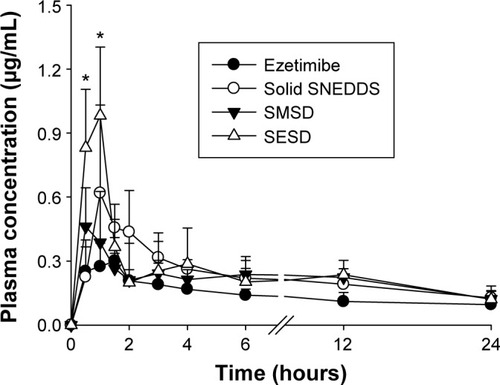
Figure 10 Plasma concentration–time profiles of ezetimibe after oral administration of various formulations in rats.
Notes: The solid SNEDDS was composed of silicon dioxide and liquid SNEDDS (1.5:3, w/v), which consisted of ezetimibe/Capryol 90/Cremophor EL/Tween 80 at a ratio of 5:10:35:55 (w/v/v/v). The SMSD or SESD was composed of the drug, HPC, and Tween 80 at a weight ratio of 3/1.5/1.5. Each value represents the mean ± SD (n=6). *P<0.05 when compared with the drug powder.
Abbreviations: SNEDDS, self-nanoemulsifying drug delivery system; SMSD, surface modified solid dispersion; SESD, solvent evaporated solid dispersion; SD, standard deviation; HPC, hydroxypropylcellulose.