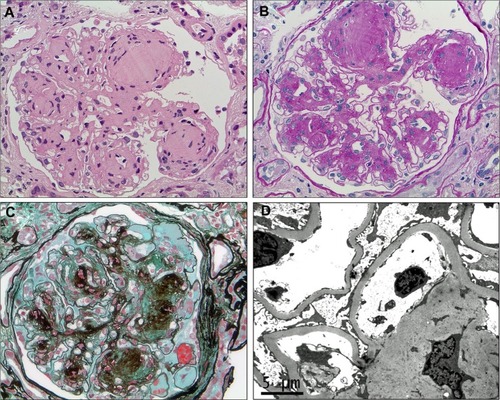
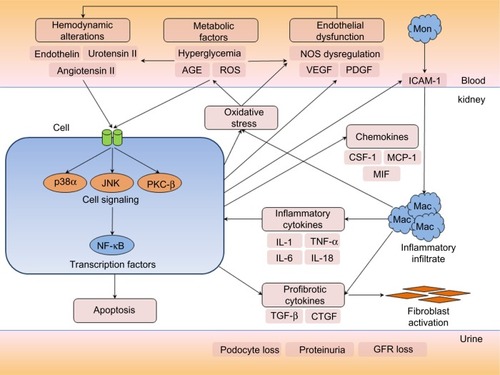
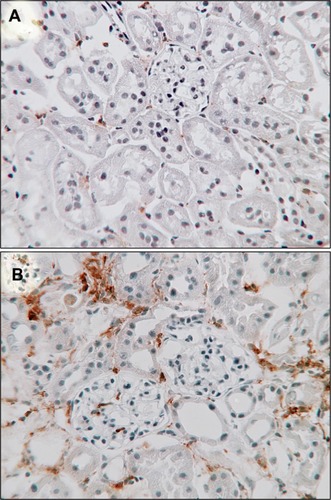
Figures & data
Table 1 Tubular biomarkers
Table 2 Summary of pharmacological treatment of diabetic nephropathy
Table 3 Diet and alternative medicine
Table 4 Summary of novel agents
Table 5 Stem cell therapy in experimental diabetic nephropathy