Figures & data
Figure 1 (A–E) Clinical manifestations of hereditary leiomyomatosis and renal cell carcinoma (HLRCC): leiomyomas.
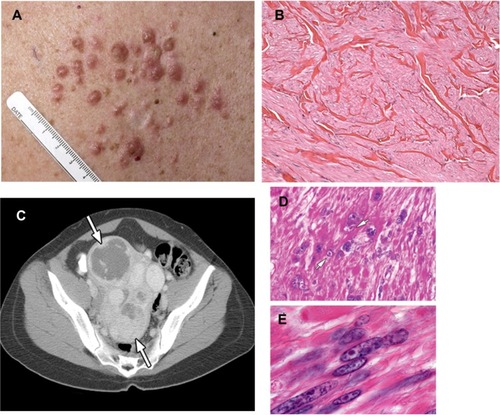
Figure 2 (A–E) Clinical manifestations of hereditary leiomyomatosis and renal cell carcinoma (HLRCC): renal tumors.
Abbreviation: RCC, renal cell carcinoma.
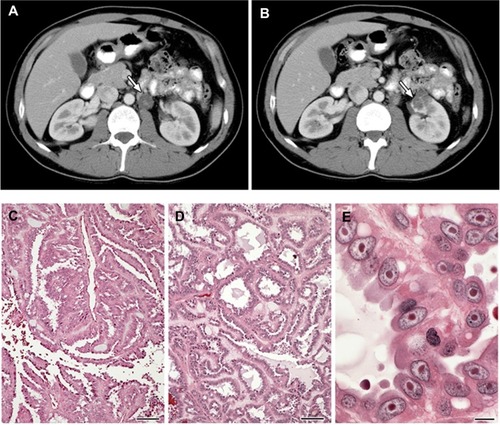
Table 1 Diagnostic criteria for hereditary leiomyomatosis and renal cell carcinoma (HLRCC)
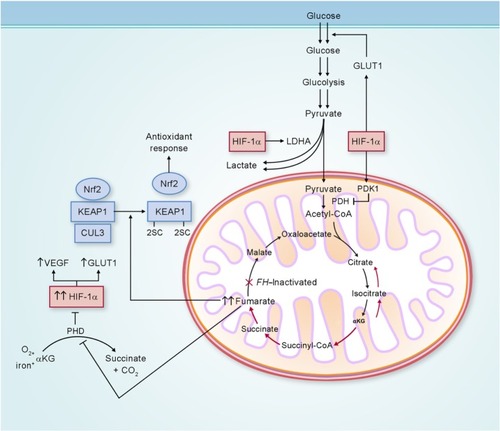
Figure 3 Potential biochemical pathways deregulated as a consequence of FH inactivation.
Abbreviations: LDHA, lactate dehydrogenase A; CoA, coenzyme A; αKG, α-ketoglutarate; FH, fumarate hydratase; GLUT1, glucose transporter 1; KEAP1, kelch-like ECH-associated protein 1; CUL3, cullin 3; HIF-1α, hypoxia-inducible factor 1 alpha; VEGF, vascular endothelial growth factor; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1.