Figures & data
Figure 1 The Sonata treatment device, combining an intrauterine sonography probe with a radiofrequency ablation handpiece into a single integrated handpiece.

Table 1 Baseline Patient Characteristics
Table 2 Baseline PBAC and Fibroid Characteristics, Full Analysis Set (N=122)
Table 3 Change in PBAC Score by Visit
Figure 2 Improvements in uterine fibroid symptom and quality of life questionnaire subscales through 12 months in the FAS population (mean values). The SSS subscale demonstrates symptom reduction whereas the HRQoL subscale denotes increases in health-related quality of life. The results on both subscales signify improvement (all P<0.0001).
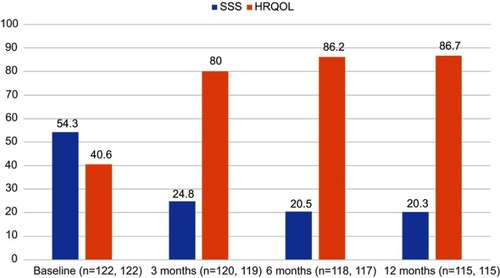
Table 4 Attributes of Ablated Fibroids*
Table 5 Outcomes of the Full and US-only Cohorts of the SONATA Pivotal IDE Trial
Table S1 List of each Institutional Review Board (IRB)
