Figures & data
Table 1 Summary of Demographic and Baseline Characteristics of Participants Who Were Randomly Assigned (n = 348)
Figure 1 Flow of participants through the trial. EE/DRSPes = ethinylestradiol 20 mcg + drospirenone 3 mg in an extended regimen. EE/DRSP24/4 = ethinylestradiol 20 mcg + drospirenone 3 mg in a 24/4 regimen.
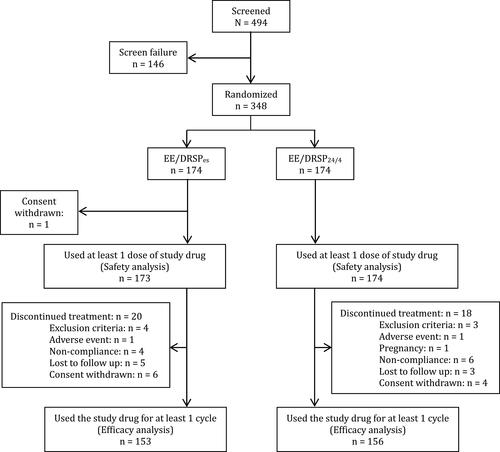
Table 2 Median Number of Days During the Entire Treatment Period (ITT, n = 309) Without Bleeding/Spotting, with Bleeding/Spotting, and with Bleeding of Each Intensity Level
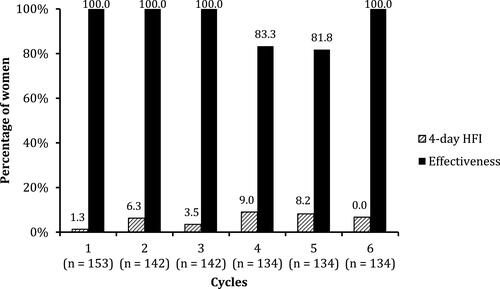
Figure 2 Number of days in the total treatment period (ITT, n = 309) with spotting only or bleeding only.
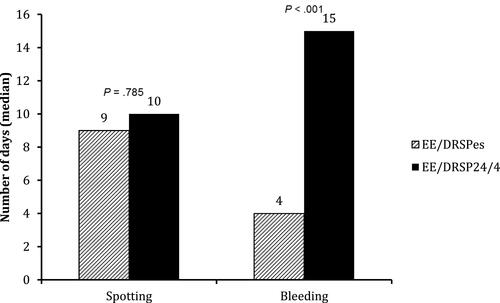
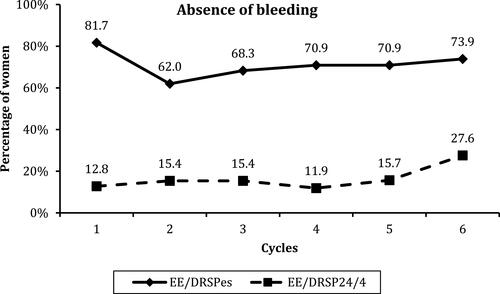
Figure 3 Numbers of days with bleeding or spotting, spotting only, and bleeding only in the 2 90-day reference periods in both groups. (A) Reference Period 1 (RP 1); (B) Reference Period 2 (RP 2).
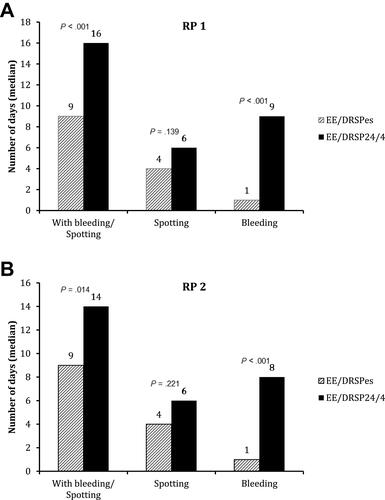
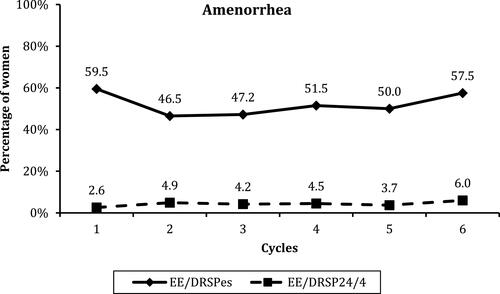
Figure 4 Percentage of women exhibiting amenorrhea (neither spotting nor bleeding) during each of the 6 28-day treatment intervals. Between-groups difference, P < 0.001.