Figures & data
Figure 1 Schematic of mode of action for VEGF signaling and bevacizumab. (A) Free unbound VEGF interacting with the receptor. (B) The monoclonal antibody, bevacizumab, binds to VEGF-A, preventing ligand-receptor interaction and downstream signaling.
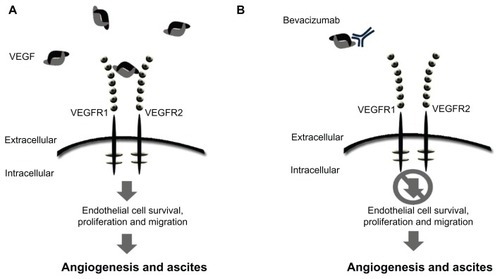
Table 1 Studies reporting use of bevacizumab for treatment of ascites associated with ovarian cancer
Figure 2 Schematic of VEGF-trap mode of action. VEGF-trap is a fusion protein that prevents VEGF-receptor binding. (A) VEGF-trap, or aflibercept, incorporates the second binding domain of the VEGFR-1 receptor and the third domain of the VEGFR-2 receptor. (B) This chimeric protein has a high VEGF binding affinity, preventing VEGF-receptor interaction and downstream signaling.
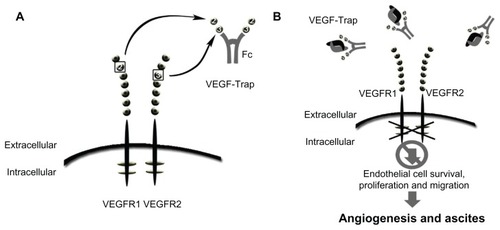
Figure 3 Schematic of mode of action of catumaxomab. (A) Catumaxomab is a trifunctional monoclonal antibody with two different antigen-binding sites and a functional Fc domain. (B) The two specific antigen-binding sites bind to epithelial tumor cells via the epithelial cell-adhesion molecule (EpCAM) and to T cells via CD3, while activating Fcγ receptor I-positive, IIa-positive, and III-positive accessory cells (dendritic cells, macrophages and natural killer cells) via its functional Fc domain.
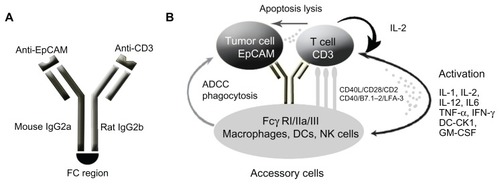
Figure 4 Kaplan–Meier plot of time to repeat paracentesis in patients treated with VEGF-trap (aflibercept) for the management of malignant ascites. Median time to repeat paracentesis was 76.0 days (95% confidence interval 64.0–178.0), which was 4.5 times longer than the baseline interval (16.8 days) in the aflibercept group.
Copyright © 2012. Elsevier. Adapted with permission from Colombo N, Mangili G, Mammoliti S, et al. A Phase II study of aflibercept in patients with advanced epithelial ovarian cancer and symptomatic malignant ascites. Gynecol Oncol. 2012;125(1):42–47.Citation53
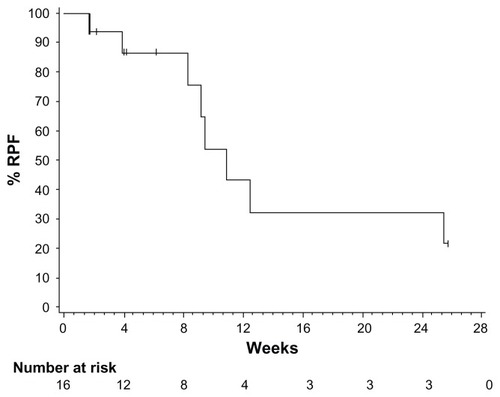
Table 2 Summary of efficacy endpoints
Figure 5 Kaplan–Meier estimates of puncture-free survival and time to next paracentesis in both the catumaxomab and control populations. (A) Puncture-free survival in the pooled population. (B) Time to next paracentesis in the pooled population.
Copyright © 2010. John Wiley and Sons. Adapted with permission from Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized Phase II/III trial. Int J Cancer. 2010;127(9):2209–2221.Citation57
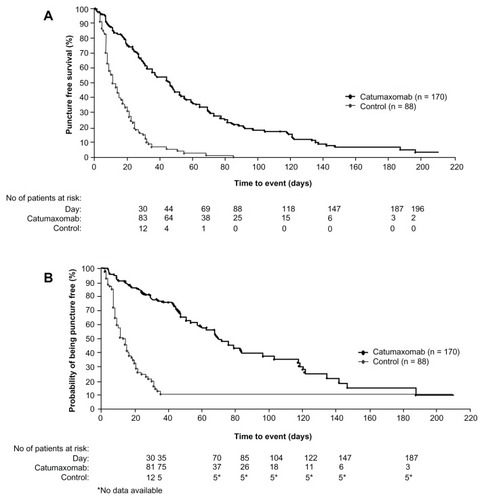
Table 3 Summary of adverse events related to catumaxomab occurring in ≥5% of subjects