Figures & data
Figure 1 Representative vaginal ring designs. (A) Over-molded metal spring design first described in a 1970 patent.Citation4 (B) Matrix-type ring with solid micronized drug dispersed throughout the entire polymer. (C) Full length reservoir/core ring design, where the drug-loaded core is encapsulated by a nonmedicated, rate-controlling polymer membrane. (D) Multiple partial core ring design, where each core contains a different drug substance. (E) Insertable core ring design, where drug-loaded cores are inserted into a prefabricated ring body before sealing the ends. (F) Sandwich or shell ring design, where a drug-loaded layer is sandwiched between a nonmedicated polymeric central core and a nonmedicated outer rate-controlling polymer membrane.
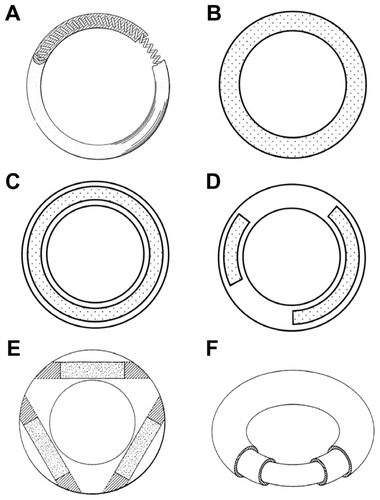
Figure 2 Chemical structures representing (A) silicone, (B) polyurethane and (C) poly(ethylene-co-vinyl acetate) materials used in the fabrication of drug-releasing vaginal rings.
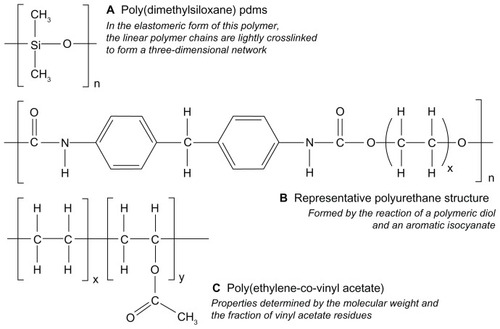
Figure 3 Schematic diagrams (to scale) describing the design and dimensions of dapivirine-releasing vaginal rings. The design in (A) was evaluated in clinical study IPM 001 () and reported in Romano et al.Citation24 The design in (B) was evaluated in IPM 008 and IPM 018 (), and reported in Romano et alCitation24 and Nel et al.Citation25 The design in (C) is the 25 mg dapivirine matrix ring due to progress to Phase III in 2012 as part of study MTN 020 (). It was previously tested in IPM 013, IPM 018, IPM 024, and IPM 027 (), and has been reported in Nel et al.Citation25 The design in (D) is the reservoir-type dapivirine-releasing ring first reported in the literature by Malcolm et al.Citation23
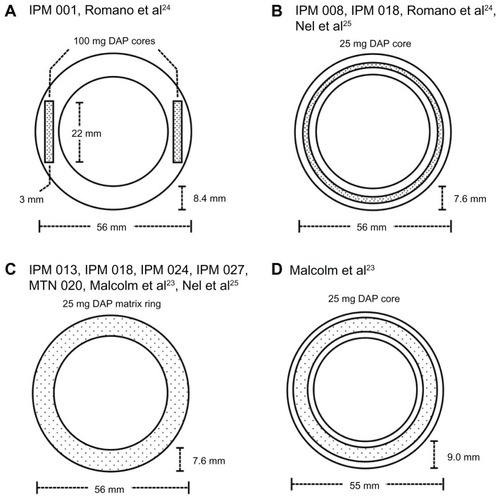
Table 1 Completed, ongoing and planned microbicide ring clinical studies
Figure 4 Reported pharmacokinetic profiles for the 25 mg matrix and 25 mg reservoir rings tested in IPM 018. (A and B) show plasma concentrations (conc) over 24 hours and 33 days, respectively. (C and D) show vaginal-fluid concentrations over 24 hours and 33 days, respectively.
© 2009 Lippincott Williams and Wilkins. Reprinted with permission. Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women.Citation25
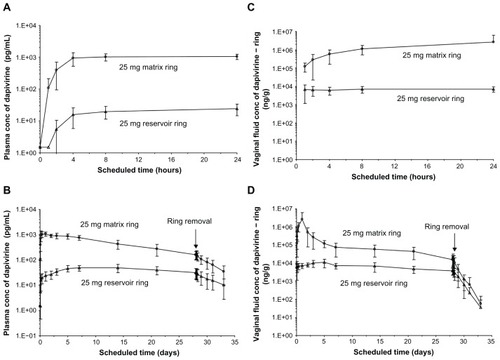
Table 2 Summary of microbicide-releasing vaginal ring devices either previously evaluated or under ongoing investigation
Figure 5 The SILCS diaphragm. (A) The thermoplastic spring core consists of nylon-6 in the original SILCS device and polyoxymethylene in the dapivirine-releasing device. (B) Spring core with over-molded silicone elastomer spacers, for positioning the spring core within the injection molds during the final overmolding step. (C) Finished diaphragm device, formed by overmolding the spring core + spacers with silicone elastomer. (D) Design features of the SILCS diaphragm. (E) Photograph of the SILCS diaphragm.
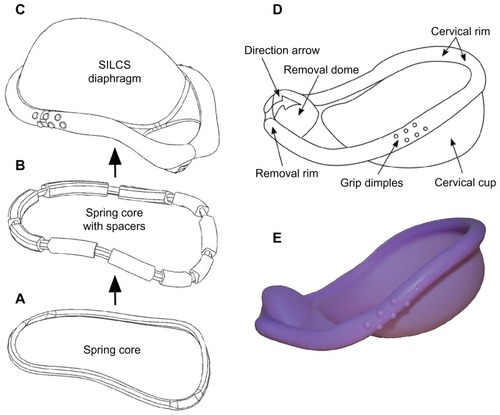
Figure 6 General manufacturing method for fabricating drug-loaded silicone elastomer matrix rings. More complex methods are required for advanced ring designs.
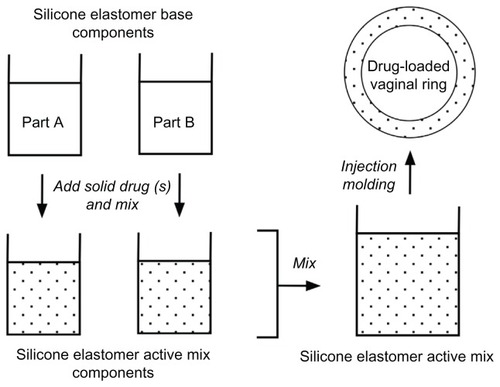
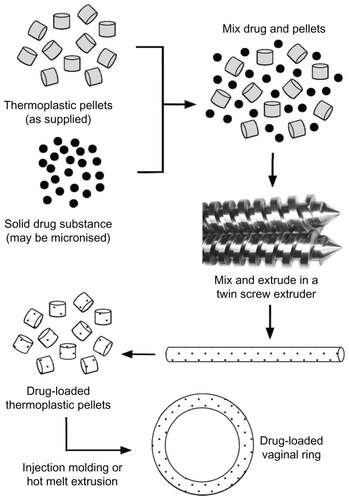
Figure 7 General manufacturing method for the fabrication of drug-loaded thermoplastic matrix rings. More complex methods are required for advanced ring designs.