Figures & data
Figure 1 Para/intracervical block injection sites.
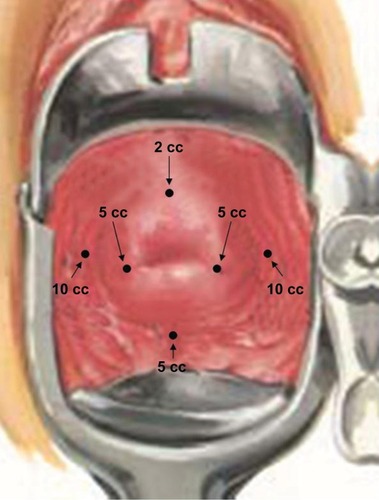
Figure 2 Intracervical block injection sites.
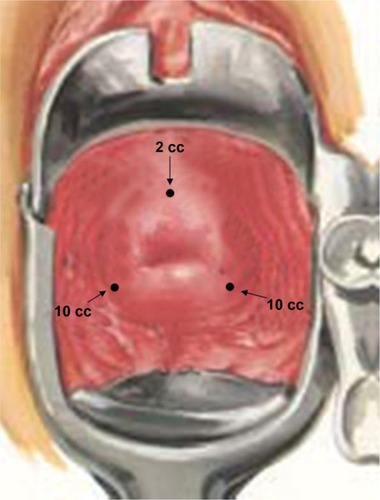
Figure 3 Hysteroscopic image of the MyoSure® tissue removal device in the uterine cavity.
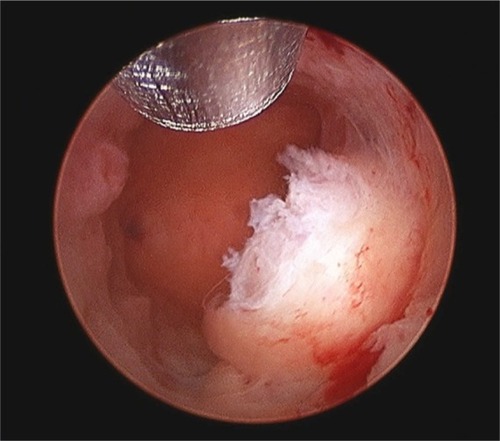
Table 1 Subject demographic characteristics and gynecological history
Table 2 Summary of lesions, by hysteroscopic evaluation
Table 3 Procedure-related and postprocedure recovery pain scores
