Figures & data
Table 1 Single-arm prospective trials: amenorrhea rates after treatment with the NovaSure® endometrial ablation device (Hologic, Inc, Bedford, MA, USA)
Table 2 Single-arm prospective trials: surgical reinterventions after treatment with the NovaSure® endometrial ablation device (Hologic, Inc, Bedford, MA, USA)
Figure 1 Randomized controlled trials: amenorrhea rates at 12 months.
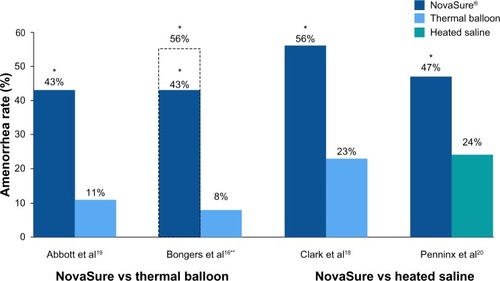
Figure 2 Randomized controlled trials: hysterectomy rates at 12 months.
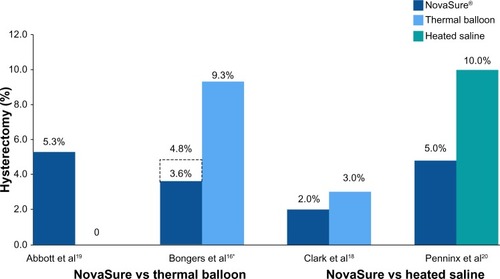
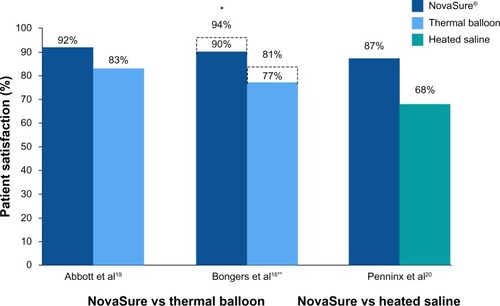
Figure 3 Randomized controlled trials: patient satisfaction at 12 months.