Figures & data
Figure 1 Subject disposition during the study.
Abbreviations: N, total number of subjects; EE, ethinylestradiol; DSG, desogestrel; FAS, full analysis set
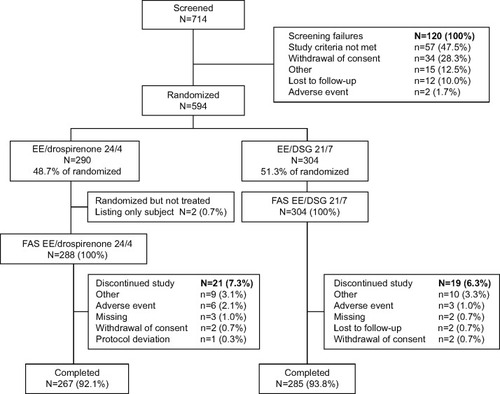
Table 1 Subject demographics at baseline and the regional analysis groups (full analysis set)
Figure 2 Change in composite symptom scores (headache + pelvic pain + bloating) during cycle days 22–28 from baseline to cycle 2 or 4 in all countries, and by regional subgroups (full analysis set).
Abbreviations: EE, ethinylestradiol; DSG, desogestrel.
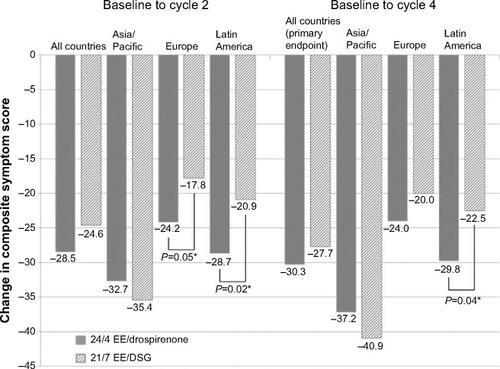
Figure 3 Change in individual symptom scores for headache, bloating, pelvic pain, and bloating and pelvic pain combined during cycle days 22–28 from baseline to cycle 2 or 4 in all countries (full analysis set).
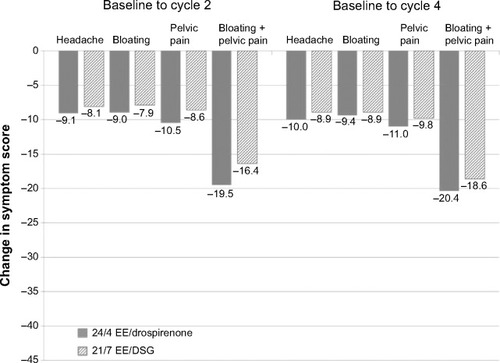
Figure 4 Change in the maximum intensity of individual symptom scores for headache, bloating, pelvic pain, and bloating and pelvic pain combined during cycle days 22–28 from baseline to cycle 2 or 4 in all countries (full analysis set).
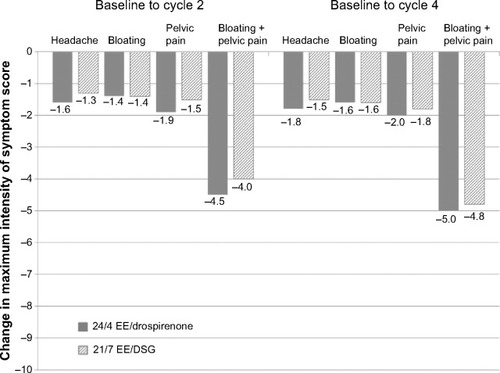
Table 2 Overview of bleeding pattern indices (90-day reference periods; full analysis set)
Table 3 Overview of AEs (safety analysis set)
