Figures & data
Figure 1 This pedigree displays four generations of relations to the patient who is indicated with the black arrow in IV-8. The family reported three infantile deaths due to undiagnosed febrile illness (IV-7, IV-9, IV-11), IV-4 has recurrent febrile illness with seizures. The pedigree shows six consanguineous marriages one in the first generation (I-1 and I-2), and five in the third generation (III-1 and III-2), (III-3 and III-4), (III-7 and III-8), (III-10 and III-11), and (III-12 and III-13). This pedigree is created using (https://progenygenetics.com) website.
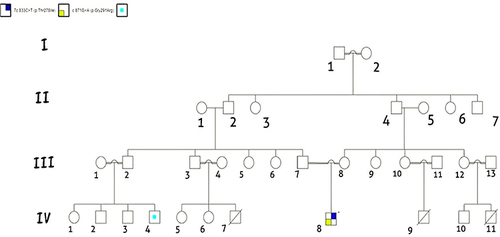
Table 1 This Tables Shows the Hematological and Immunophenotyping Results for the Patient
Figure 2 This is a schematic structure of the affected sites of ZAP70 protein. ZAP70 protein domains are the amino-terminal SH2 domain (N-SH2), interdomain A, carboxy-terminal SH2 domain (C-SH2), interdomain B and lastly the kinase domain of the protein. The tandem SH2 domains interact with the doubly phosphorylated tyrosine-based activation motif of CD247/CD3Z. Also, both mutations in this case occur in the interdomain B. The interdomain B region contains three tyrosine (Tyr-292, Tyr-315, Tyr-319) that are phosphorylated after T cell receptor activation. Interestingly, (p.Gly291Arg) mutation is immediately next to Tyr-292 and it may impact the ZAP70 protein as resulted in this case, notable (p.Thr278Ile) and (p.Gly291Arg) mutations are on opposite chromosomes.
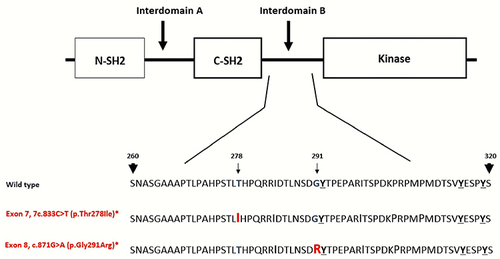
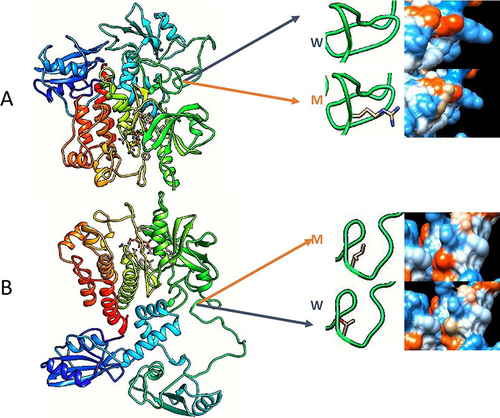
Figure 3 This is a molecular visualizations of ZAP70 protein implicated in this case, (A) shows the c.871G>A (p.Gly291Arg) mutation. (B) shows 7c.833C>T (p.Thr278Ile) mutation. The (W) letter represent the wild type while (M) represent mutant type in both figures. Due to mutations, amino acid hydrophobicity may changed in the ZAP70 protein, and it is shown next to the amino acid in both figures. Notably, from SIFT, Align-GVGD, and PolyPhen-2 algorithms, only the later predicted that (p.Gly291Arg) mutation is “Possibly Damaging”, and also predicted that other mutation is “Benign”. We created this illustration using Swiss-MODEL (https://swissmodel.expasy.org) and Chimera 1.16 software after mutating the ZAP70 with the patient’s ZAP70 mutations.
Data Sharing Statement
Data that support this case presentation are available upon contacting the corresponding author.
