Figures & data
Table 1 Laboratory Test Results of the Patient with Chlamydia Psittaci Pneumonia and SARS-CoV-2 Coinfection
Figure 1 Chest computed tomography. (A–C) Chest computed tomography (CT) on admission showing massive consolidation in the left lower lung field and left-sided pleural effusion. (D–F) Chest CT after 1 week of treatment with moxifloxacin, showing a reduction in the consolidation, lung lesions, and pleural infusion. (G–I) Chest CT after 2 weeks of treatment with moxifloxacin.
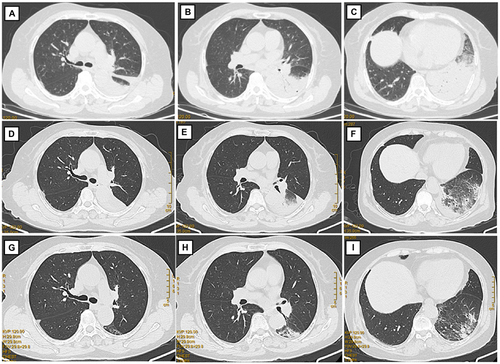
Figure 2 Detection depths and coverage of SARS-CoV-2 Omicron XBB.1 and Chlamydia psittaci in different sample types. (A and B) Detection depths and coverage of SARS-CoV-2 Omicron XBB.1 in (A) BALF and (B) nasopharyngeal swab specimens. (C and D) Detection depths and coverage of C. psittaci in (C) BALF and (D) nasopharyngeal swab specimens.
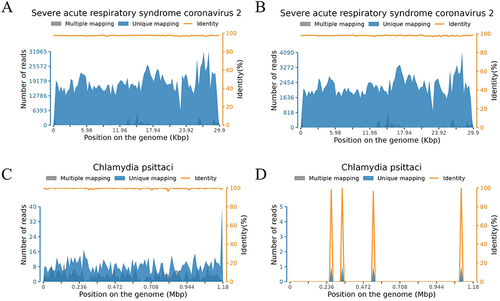
Table 2 C. Psittaci and COVID-19 PCR Primers Used in This Study
Table 3 mNGS and Confirmative qPCR Results
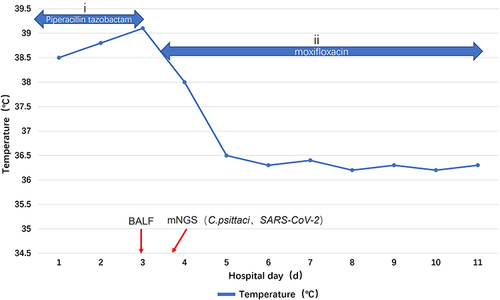
Figure 3 The patient’s clinical course and treatment. Body temperature alterations and antibacterial therapy, (i) 4.5 g piperacillin-tazobactam administered as an intravenous bolus every 8 hours on days 1–3 and (ii) 0.4 g of moxifloxacin administered as an intravenous bolus once daily on days 4–11.