Figures & data
Table 1 Inclusion and exclusion criteria
Figure 1 Flow chart of study participants.
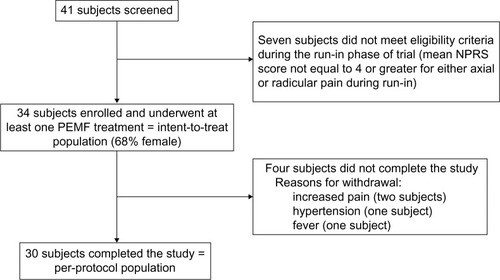
Table 2 Subject baseline characteristics
Figure 2 Mean weekly numerical pain rating scores for responders.
Abbreviation: NPRS, numerical pain rating scale score.
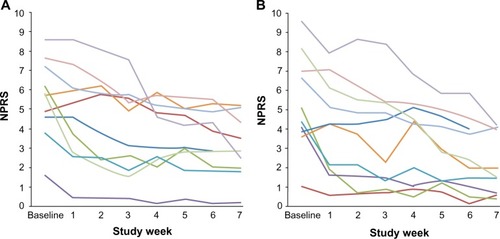
Table 3 Responder analysis for complete per-protocol population and procedure-based subpopulations
Table 4 Percentage of subjects with clinically meaningful outcomes (end of treatment versus baseline)
Table 5 Treatment-emergent adverse events
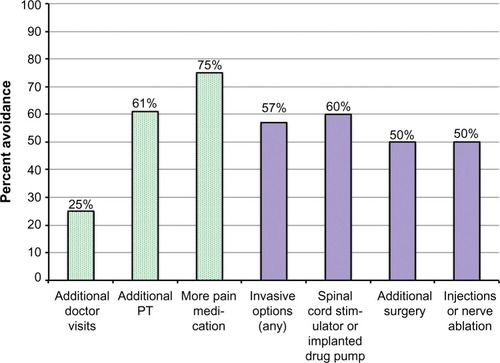
Figure 3 Cost-avoidance survey results for per-protocol survey respondents (28/30).
Abbreviation: PT, physical therapy.