Figures & data
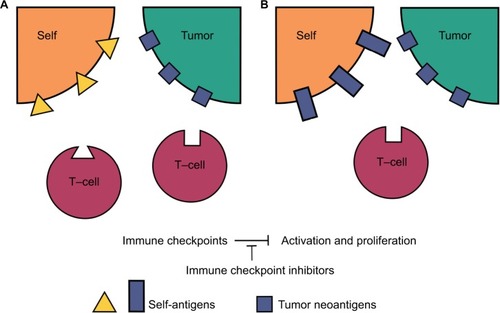
Figure 1 Peripherally tolerized T-cells and their target antigens in irAEs.
Notes: In both tumor environments, neoantigens caused an anti-tumor immune response, which has subsequently been downregulated through immune checkpoint pathways. (A) Self-reactive peripheral T-cells have also been tolerized through immune checkpoints. Treatment with immune checkpoint inhibitors in this environment will lead to activation and upregulation of both the anti-tumor and an autoimmune reaction. If the tumor-specific response is weak or nonexistent in this setting, the autoimmune irAE will appear in isolation without a corresponding tumor response. (B) The tumor-reactive tolerized T-cell not only displays affinity for tumor neoantigens, but also for similar self-antigens on healthy tissue. Immune checkpoint inhibitor treatment in this environment will lead to a cross-reactivity irAE in addition to a tumor-specific response. If the tumor-specific response is weaker than the cross-reactive response, it may also appear that and irAE has occurred in isolation in this setting.
Abbreviation: irAE, immune-related adverse event.
Abbreviation: irAE, immune-related adverse event.