Figures & data
Table 1 Activating Receptors on NK Cells and Their Targeting Approach for Anti-Tumor Immunity
Figure 1 Activating and inhibitory receptors on NK cells and their signaling pathway. NK cells express various activating and inhibitory receptors. Upon interaction with their cognate ligands on the target (tumor) cell surface, they initiate a downstream signaling pathway that ultimately dictates the effector and cytotoxicity function of NK cells. The interaction of the activating receptor with their ligand initiates primarily mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K)/protein kinase B (Akt)/Mammalian target of rapamycin (mTOR) pathway that activates nuclear factor- κB (NFκB) transcription factor. Activating receptors also activate the Ca2+ signaling pathway through phospholipase Cγ (PLCγ)-protein kinase C (PKC) axis that further activates the nuclear factor of activated T cells (NF-AT) transcription factor. Activated NF-κB and NF-AT drive the production of cytokines and regulate the cytotoxic functions of NK cells. In contrast, the interaction of inhibitory receptors with their ligand activates the Src homology region 2 domain-containing phosphatase-1 (SHP-1) that inhibits the functions of downstream signaling kinases and inhibits the activation of transcription factors like NF-κB and concurrently inhibits NK cell effector and cytotoxic function.
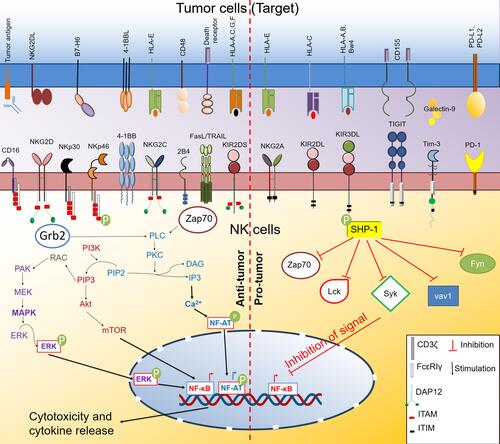
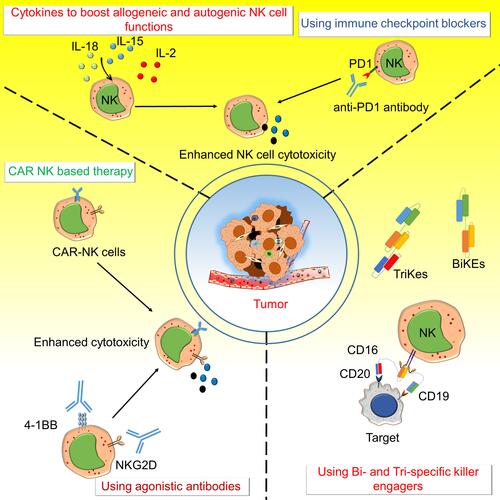
Figure 2 Various approaches to target NK cell activating and inhibitory receptors for anti-tumor immunity. The activating and inhibitory receptors on NK cells are targeted by several different strategies like Chimeric antigen receptors – natural killer (CAR-NK) cells, bi-specific killer engagers (BiKEs), and tri-specific killer engagers (TriKEs), agonistic antibody to NKG2D and 4–1BB, immune checkpoint blockers like an anti-PD1 antibody, and using various cytokines to boost allogenic and autogenic or iPSC derived NK cells functions to boost anti-tumor immunity. CARs on NK cells were developed to target various molecules expressed on cancer cells to direct the NK cell-mediated killing of cancer cells. BiKEs and TriKEs mainly engage some tumor antigens with Fc receptors (CD16/CD32) of NK cells and enhance cytotoxic activity. Various agonistic antibodies target the activating receptors such as NKG2D and 4–1BB on NK cells and antagonistic antibodies of immune checkpoint molecules like anti-PD1 to enhance anti-tumor activity. Different cytokines (IL-2 IL-15 and IL-18) are also used to increase the NK cell survival and activity.