Figures & data
Figure 1 Drawing of the structure of the anatomy of human skin. The subcutaneous space contains cells and extracellular matrix, within which are such structural components such as collagen and elastin fibers that support blood and lymphatic vessels. All of the cells and vessels and the structural–scaffold proteins collagen and elastin are embedded in a gel-like substance made up of glycosaminoglycans and proteoglycans. When a subcutaneous injection is given, small molecules are absorbed through the capillary endothelium; however larger molecules, such as antibodies, are excluded from capillaries and enter through the larger pores of the fenestrated lymphatic endothelium.
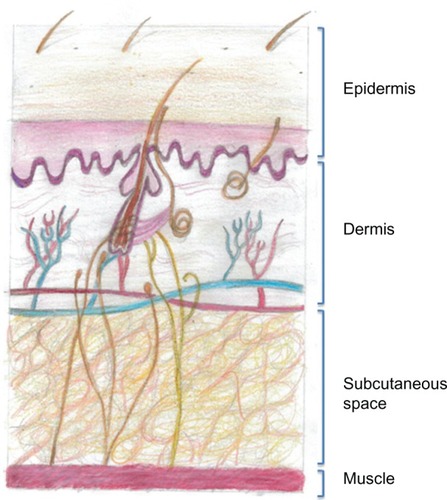
Table 1 Differences between routes of administration
Figure 2 Immunoglobulin (Ig) pharmacokinetics for intravenous (IVIg), subcutaneous (SCIg), and facilitated SCIg (fSCIg) administration. The graph shows an illustrative representation (not patient data) of the differences in the levels of IgG in the blood following IVIg in blue and fSCIg infusion in red, both given as a first infusion, compared with conventional SCIg in green, which is shown at a steady state over a 28-day time period. This shows the differences in the pharmacokinetics with the three methods of delivery, illustrating the loss of the peak level achieved with IVIg when fSCIg is used, followed by (from around day 12) the very similar gradual decline in IgG levels over time. SCIg is not represented from initiation of treatment, as this would take 3–6 months to reach a steady state unless an SCIg-loading regimen was employed.
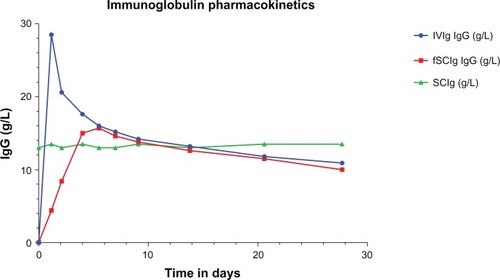
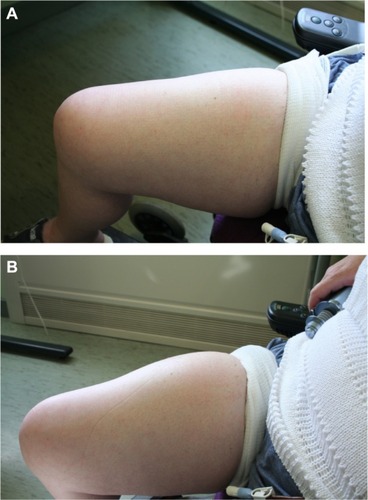
Figure 3 Skin pre- (A) and post- (B) hyaluronidase-facilitated subcutaneous immunoglobulin infusion (fSCIg) after 3 1/2 years of fortnightly infusions. Images of the thigh taken before and after the infusion of 130 mL of 16% Subcuvia (Baxter, Deerfield, IL, USA) at 100 mL/hour.Citation36 These show a diffuse swelling over a larger area than conventional SCIg with an absence of blanching and erythema, as the fluid is more widely distributed in the SC space. The initial SC injection of ovine hyaluronidase at a dose of 50 U/g of IgG was immediately followed by the Ig infusion. Reproduced from Journal of Clinical Pathology, Knight E, Carne E, Novak B, et al., 63(9), 846-847, 2010 with permission from BMJ Publishing Group Ltd.