Figures & data
Figure 1 Magnifying glass on the mechanism of action of apremilast, simplified and schematic representation. In monocytes and dendritic cells, cAMP is degraded to AMP mainly by PDE4. PDE4 inhibition by apremilast increases intracellular cAMP levels and determines the activation of PKA. PKA activation induces the phosphorylation of transcription factors such as CREB. These transcription factors also bind to sites within promoters of IL-10, increasing IL-10 expression. Co-activators are also involved resulting in the inhibition of NF-κB transcriptional activity and expression of IL-23, TNF-α, and IFN-γ. The decreased production of inflammatory mediators reduces cellular infiltration in the target tissue and the activation and proliferation of keratinocytes and synoviocytes in the psoriatic skin and synovium.
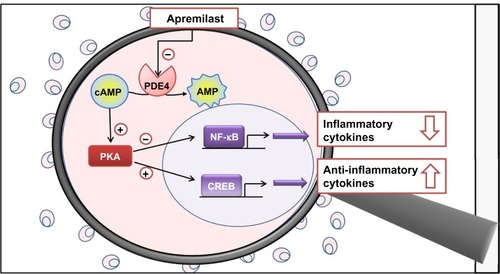
Table 1 Detailed preliminary results of ongoing randomized double-blind controlled trials PALACE 2, 3, and 4 at primary endpoint (week 16, placebo-controlled) and long-term (52 weeks) data, which include patients originally randomized to apremilast and completing 52 weeks of study
