Figures & data
Figure 1 Illustration depicting the associations between neurons and glial cells in the CNS. (The CNS is primarily made up of neurons and glial cells, including astrocytes, oligodendrocytes, and microglia. Glial cells play a crucial role in supporting the survival of neurons by providing them with trophic factors).
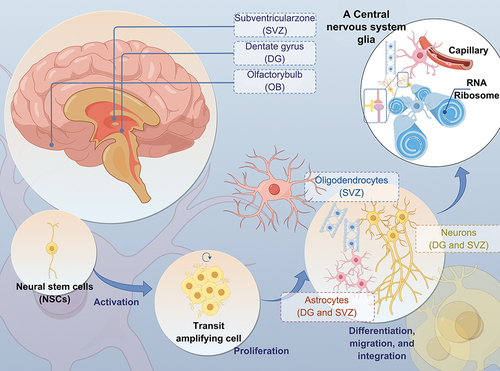
Figure 2 Microglia Function and Communication (Microglia play essential roles in the CNS, such as synaptic pruning, synaptogenesis, axon fasciculation, neurite formation, programmed cell death, astrocyte activation and proliferation, and oligodendrocyte differentiation and myelogenesis).
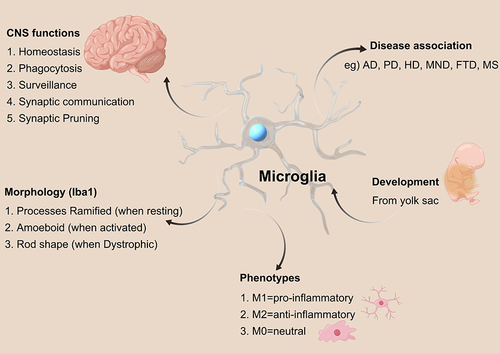
Figure 3 Impact of microglia on AD. (Healthy and young cells, such as microglia, release pro- and anti-inflammatory mediators, growth factors, and bioenergetic pathway mediators. These substances play a role in maintaining brain homeostasis, supporting neuronal survival, and preserving cognitive function).
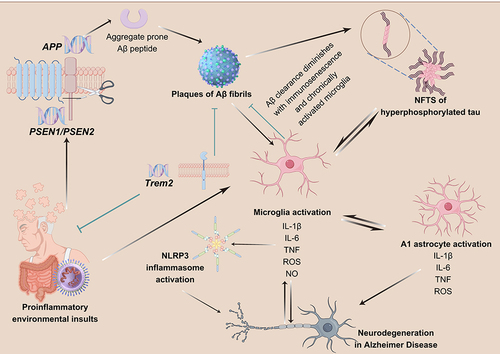
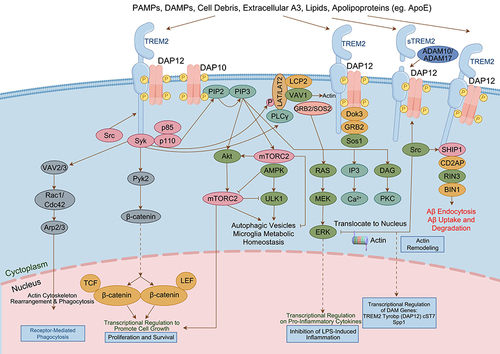
Figure 6 Function of TREM2 in AD.Citation90 (TREM2 expression plays a crucial role in regulating the proliferation, migration, and phagocytosis ability of microglia. In the early stages of AD, microglia expressing TREM2 effectively surround and interact with lipidated Aβ plaques to clear them, thus preventing the spread of Aβ. However, in the late stages of AD, these TREM2-expressing microglia struggle to clear the aggregates, leading to chronic inflammation, reduced phagocytosis ability, and the induction of tau phosphorylation and aggregation. Individuals with TREM2 mutations experience weakened TREM2 affinity, resulting in impaired microglial proliferation and migration, which hinders the effective clearance of Aβ aggregates. Furthermore, TREM2 mutations lead to increased cytokine secretion from microglia, ultimately exacerbating the spread of Aβ and the phosphorylation and aggregation of tau, thereby worsening AD pathology).
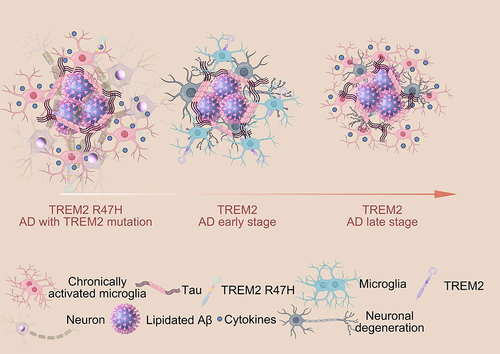
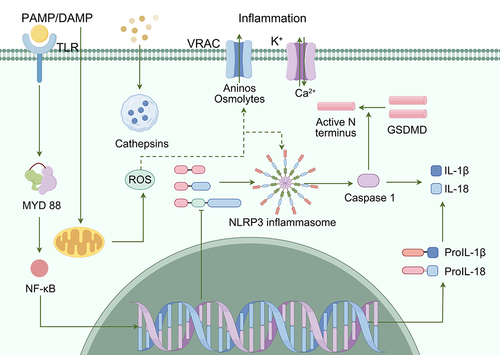
Figure 7 The role of NLRP3 inflammasome in AD.Citation147 (Microglia are activated in response to DAMPs from Aβ plaques, and are mobilized to eliminate them. However, prolonged activation of microglia and immunosenescence can reduce their effectiveness over time. TREM2 plays a role in regulating the activity and survival of microglia. Mutations in TREM2 can affect the ability of microglia to regulate cytokine production and clear neural debris. Failure to clear Aβ plaques can lead to the formation of NFTs, triggered by both the plaques and the persistently activated microglia. Additionally, hyperphosphorylated tau protein can further stimulate microglia.