Figures & data
Figure 1 The phenotypic profile and potential cellular origins of induced (i)Treg present in the tumor microenvironment.
Abbreviations: Treg, T regulatory cells; nTreg, näive Treg; tTreg, thymus-derived Treg; iTreg, inducible Treg; pTreg, peripheral Treg; 5′AMP, adenosine-5′-monophosphate; ADO, adenosine; CTLA-4, cytotoxic lymphocyte antigen-4; PD-1, programmed death-1; TIM-3, T cell immunoglobulin mucin-3; LAG-3, lymphocyte activation gene-3; TGF-β, transforming growth factor-beta; LAP, latency-associated protein; GARP, glycoprotein A repetitions predominant; NRP-1, Neuropilin-1; ADP, adenosine diphosphate; KLF2, Kruppel-like factor 2; TCR, T cell receptor; ATP, adenosine-5′-triphosphate.
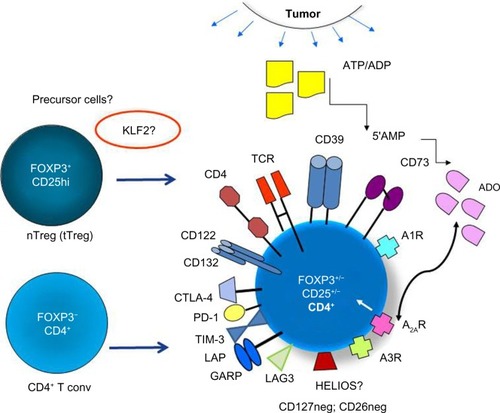
Figure 2 Treg accumulating in the TME (activated iTreg) utilize various suppressive mechanisms to inhibit functions of Teff.
Abbreviations: Treg, T regulatory cells; TME, tumor microenvironment; iTreg, inducible Treg; Teff, T effector cells; IL, interleukin; ADO, adenosine; TGF-β, transforming growth factor-beta; GrB, granzyme B; NRP-1, Neuropilin-1; ATP, adenosine-5′-triphosphate; ADP, adenosine diphosphate; TCR, T cell receptor; cAMP, cyclic adenosine monophosphate.
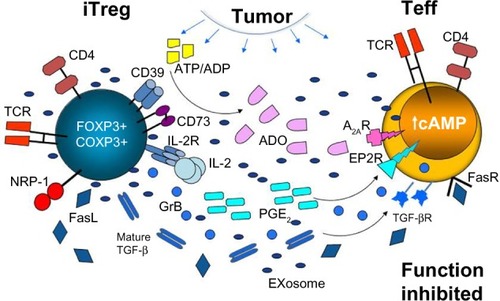
Table 1 Potential molecular targets for therapeutic depletion or re-programming of human Treg
Table 2 Immunotherapy clinical trials incorporating strategies for Treg depletion in patients with solid or hematologic malignancies
