Figures & data
Figure 1 Patient selection.
Abbreviations: COPD, chronic obstructive pulmonary disease; EIB, exercise-induced bronchoconstriction; HFA, hydrofluoroalkane; IDC, integrated dose counter; NDC, National Drug Code; SABA, short-acting beta agonist.
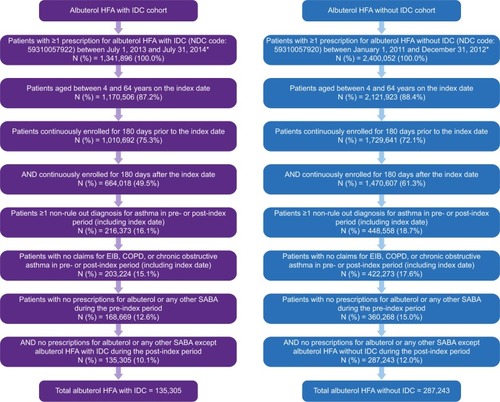
Table 1 Patient demographic and clinical characteristics, by age group
Table 2 Health care utilization among users of albuterol inhalation aerosol with and without IDC, by age group during 6 months of follow-up
Figure 2 Adjusted per-patient number of respiratory-related hospitalizations and ED visits during the 6-month follow-up period.
Abbreviations: ED, emergency department; HFA, hydrofluoroalkane; IDC, integrated dose counter.
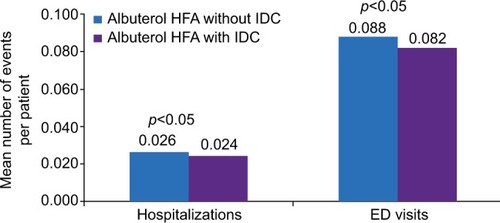
Table 3 Multivariable logistic regression odds ratios for predicting respiratory-related hospitalizations and ED visits during 6-month follow-upTable Footnotea
