Figures & data
Figure 1 Phase III conjunctival allergen challenge (CAC) study design.
Abbreviations: BBOS, bepotastine besilate ophthalmic solution; TAI, test-agent instillation.
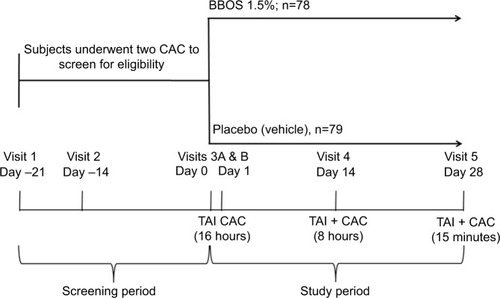
Figure 2 Phase IV environmental allergen study design.
Abbreviation: BBOS, bepotastine besilate ophthalmic solution.
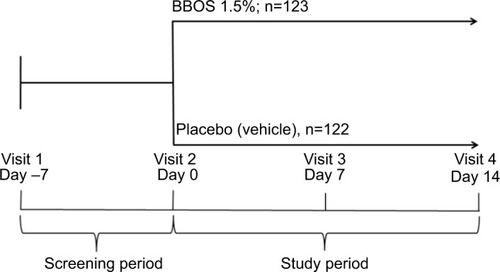
Table 1 Mean rhinorrhea, nasal congestion, nasal pruritus, and nasal ocular symptom scores post-CAC conducted at 15 minutes, 8 hours, and 16 hours after dosing
Table 2 Mean change from baseline for twice-daily averaged nasal symptom scores over the treatment period (ITT population)
Figure 3 Mean reflective sneezing scores by day and time (ITT population).
Abbreviations: ITT, intent to treat; BBOS, bepotastine besilate ophthalmic solution.
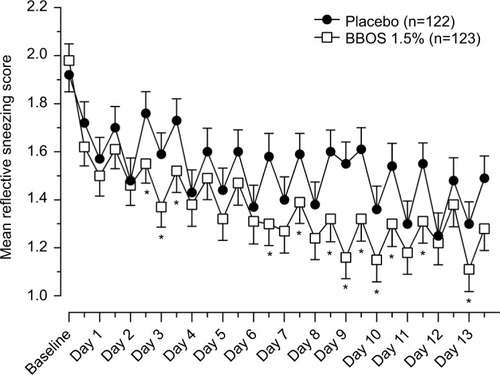
Table 3 Median days to improvement of nasal symptoms (ITT population)
Table 4 Global therapeutic response ratings by subjects and investigators (proportions)
