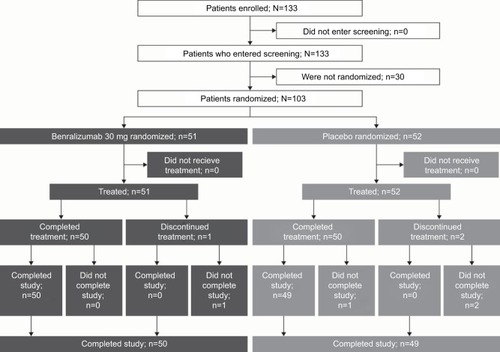
Figures & data
Figure 1 Study design.
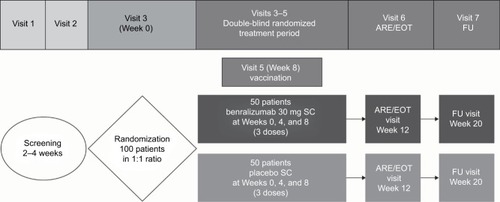
Table 1 Patient demographics and baseline clinical characteristics (full analysis set)
Figure 3 Influenza strain antibody geometric mean fold rise from Week 8 to Week 12 for (A) hemagglutination inhibition and (B) microneutralization (vaccine immunogenicity analysis set).
Abbreviation: Q4W, every 4 weeks.
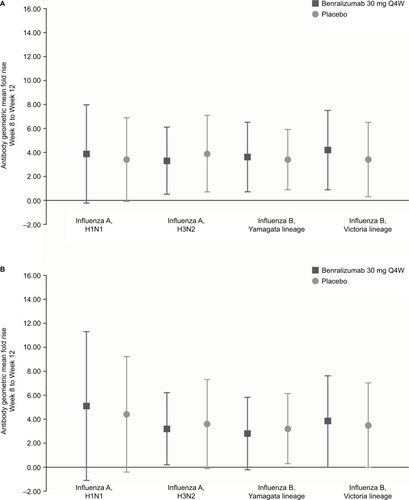
Table 2 Influenza strain antibody response for hemagglutination inhibition (vaccine immunogenicity analysis set)
Table 3 Influenza strain antibody response for microneutralization (vaccine immunogenicity analysis set)
Table 4 ACQ-6 scores by time point (full analysis set)
Figure 4 Arithmetic mean (± SD) serum trough concentrations of benralizumab (PK analysis set).
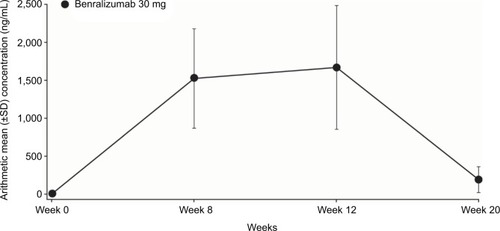
Table 5 Adverse events (safety analysis set)